The Endocrine Glands in the Dog: From the Cell to Hormone
The animal body represents one of the more complex and perfect systems of nature. Despite its complexity and its functionality, which is incredibly effective, the control of its basic functions is performed by only two systems: the nervous system and endocrine system. The nervous system is associated with electrical and chemical signals that are transmitted at high speed, resulting in rapid organic activities. The endocrine system acts through the synthesis and release of chemical messengers and is responsible for several functions of the organism, in a slower, but more durable way.
Endocrinology is the science that studies the internal secretions produced by endocrine glands. Endocrine glands are distributed throughout the body and secrete chemical messengers – hormones, in response to an internal or external stimulus. These hormones are released directly into the bloodstream – endocrine mechanism, in contrast to exocrine glands, which use a ductal system to release their secretions in locations that lead, ultimately, to the exterior of the body – exocrine mechanism.
Hormones are transported through the bloodstream to target organs, where they will exert a physiological control, even in low concentrations, coordinating a multiplicity of organic functions and maintaining homeostasis.
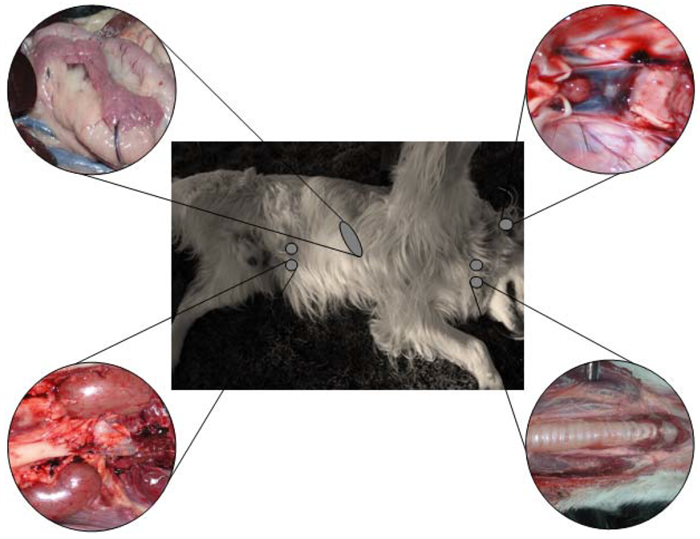
The main endocrine glands in the animal body include pituitary gland, thyroid, parathyroid, pancreas, adrenal (Figure 1), and gonads (ovaries and testes).
2. Hypothalamic-pituitary axis
The hypothalamus, located at the base of the ventral diencephalon is limited rostrally by the optic chiasm, caudally by the mammillary processes, laterally by the temporal lobes and dorsally by the thalamus. Ventrally, it continues through the infundibulum which connects it to the pituitary gland. Although not considered a real endocrine gland, the hypothalamus coordinates all pituitary activity by the release of a number of peptides and amines that control the secretion of hormones in the pituitary gland (also named hypophysis). Their terminology ends in RH that means releasing hormone (GnRH – gonadotrophin releasing hormone; CRH – Corticotrophin releasing hormone; TRH – Tirotrophin releasing hormone; PRH – Prolactin releasing hormone; GHRH – Growth hormone releasing hormone). Table 1 summarizes the major hormones produced by this hypothalamic-pituitary axis.
Table 1.
Hormones produced by the hypothalamic-pituitary axis.
The pituitary is a small oval shaped gland, lodged in the sella turcica , a small depression of the sphenoid bone, which is located at the base of the brain and positioned next to the optic chiasm (Figure 2), being connected to the hypothalamus by a funnel-shaped hypophyseal stalk [1,2].
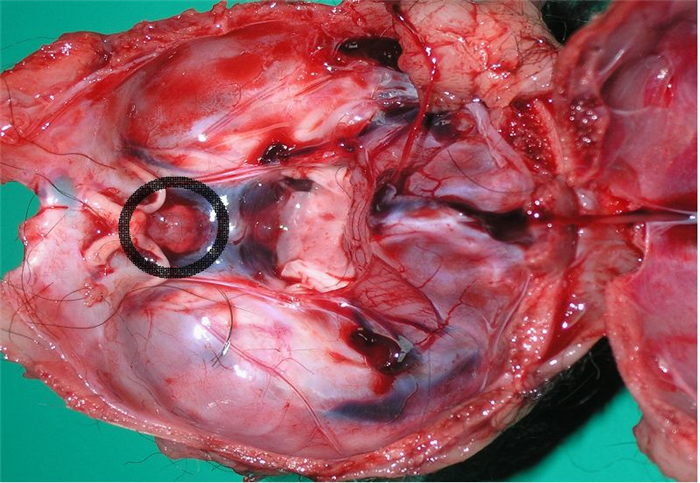
The pituitary gland is divided into two regions, the adenohypophysis, or anterior pituitary gland, and the neurohypophysis, also called the posterior pituitary gland or pars nervosa.
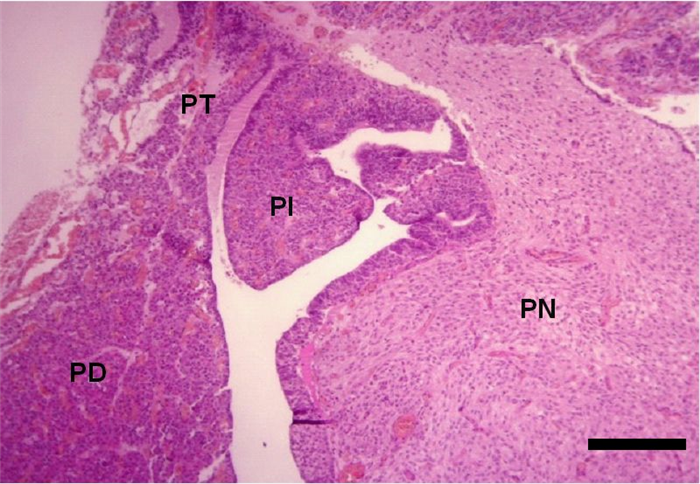
The adenohypophysis, in turn, is divided in three sections: pars distalis, pars tuberalis and pars intermedia (Figure 3) [3].
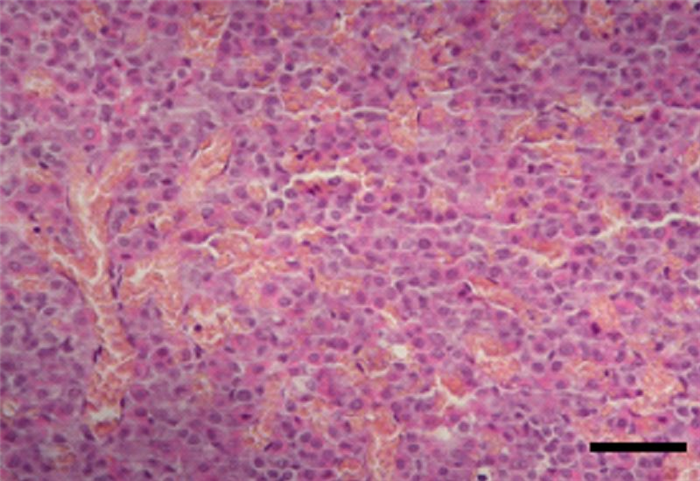
- The parenchyma of the pars distalis is composed by chromophilic cells, acidophilic or basophilic, arranged in cords, nests or follicles (Figure 4) and by small, round endocrine cells, without evident granules – chromophobe cells which means that they have low tinctorial affinity, also known as principal cells, reserve cells or C cells [4]. Chromophilic cells secrete somatotropic hormone (also known as somatotropin – STH or growth hormone – GH), prolactin (PRL), adrenocorticotropic hormone (ACTH), follicle-stimulating hormone (FSH), luteinizing hormone (LH) (which is also called interstitial cell-stimulating hormone or ICSH in males), thyrotropin or thyroid-stimulating hormone (TSH), melanocyte-stimulating hormone (MSH) and lipotropines [4]. Somatotropin promotes epiphyseal growth [6] and protein production, whereas prolactin leads to mammary gland development and milk secretion. ACTH acts on the adrenal gland cortex, resulting in an increased release of adrenocortical hormones [7]. FSH and LH are gonadotropic hormones, with different effects in males and females [8,9]. TSH acts on the thyroid gland, stimulating its secretion [10]. Lipotropines have the same precursor molecule as ACTH (propiomelanocortin – POMC) [3,5] and, after cleavage, can originate β-MSH and β-endorfine, a pain blocking agent [3] (Table 2).
Also, considering its secretion, chromophilic cells of pars distalis, may be classified in four types:
Lactotrophs or lactotropic cells which secrete PRL (acidophilic) [11].
Corticotropes or corticotropic cells which secrete ACTH (basophilic) [12].
Gonadotropes or gonadotropic cells which secrete gonadotropins LH and FSH (basophilic) [13].
Thyrotrophs or thyrotropic cells which secrete TSH (basophilic) [14].
The chromophobe cells are also arranged in clusters or cords and their low tinctorial affinity probably indicates that these cells may correspond to depleted cells of any of the above types described or a state of partial degranulation. However, some of the chromophobe cells may also be undifferentiated, nonsecretory cells [4, 15].
- The pars tuberalis is composed by cuboidal weakly basophilic cells, arranged in cords, nests or follicles and its function is not yet well established [4]. Studies suggest that one of its primary functions, in seasonal mammals (e.g. sheep), is to mediate photoperiod influences in prolactin secretion variations through an unidentified factor called tuberalin i.e. tuberalin performs hormonal control, both in gene expression and in the release of prolactin from lactotrophs in the pars distalis [3].
- The pars intermedia is more developed in domestic animals than in man, consisting of nonspecific basophilic cells, arranged mainly in cords and nests, but also in follicles (Figure 5), which secrete MSH [4] that in turn, promotes distribution of melanin granules, and therefore, darkening of skin [10].
The neurohypophysis is devoid of endocrine cells, being composed of demielinized neurons (Figure 6) whose bodies are in the supraoptic and paraventricular nuclei of the hypothalamus, constituting the storage of the hypothalamic hormones: antidiuretic hormone (ADH), also called vasopressin, oxytocin and neurophysins. These hormones are produced in the hypothalamus, transported through the hypothalamic-pituitary axis and stored in the neurohypophysis, until a stimulus induces their release [4, 16] (Table 2).
ADH has several effects on the body, particularly in terms of water saving and in increasing blood pressure. Oxytocin stimulates contraction of the myoepithelial cells of the mammary gland, causing the ejection of milk. It also binds to the smooth muscle cells of the uterus, promoting uterine contractions during parturition [10] (Table 2).
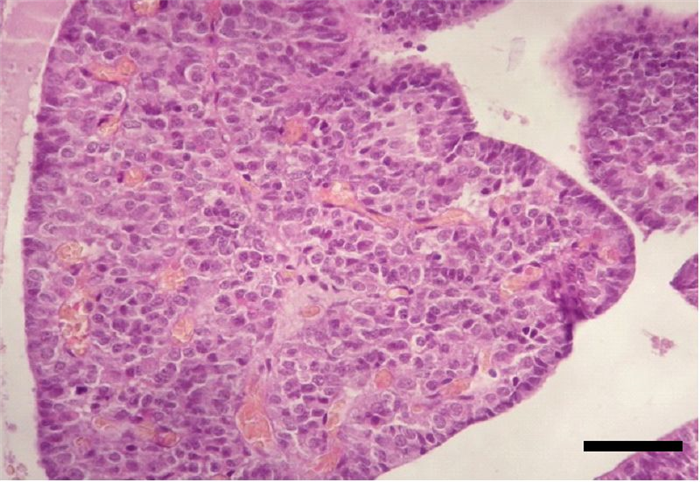
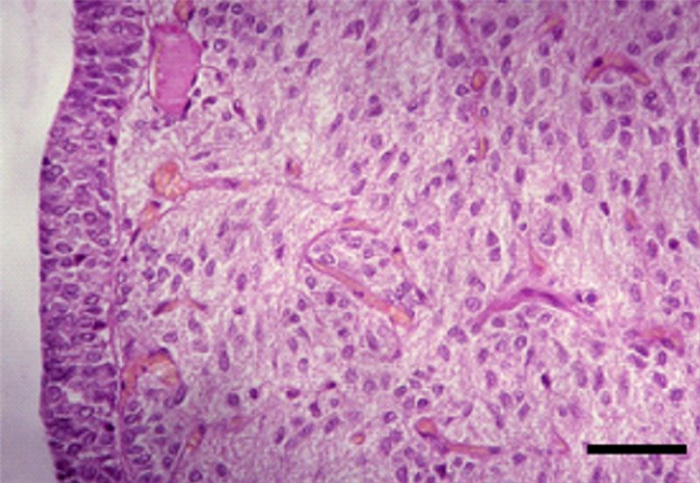
Table 2.
Hormones produced by the pituitary gland and their functions.
3. Thyroid
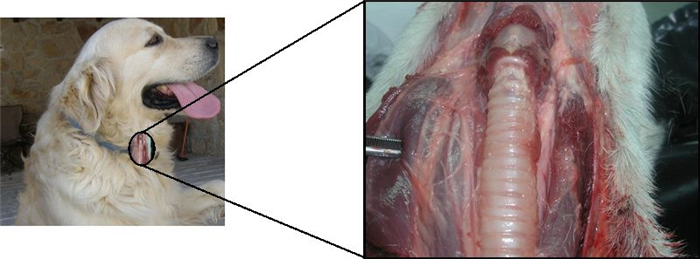
Canine thyroid gland is located at cervical ventral region, lateral and ventral to the 5 th -8 th tracheal rings (Figure 7), being composed by two separate lobes, occasionally connected by an isthmus [17, 18].
In the lobes, the majority of the epithelial cellular population (90%) ranges in height, from low cuboidal to high columnar epithelial cells, which are organized into follicles – follicular cells (Figure 8) [4, 16].
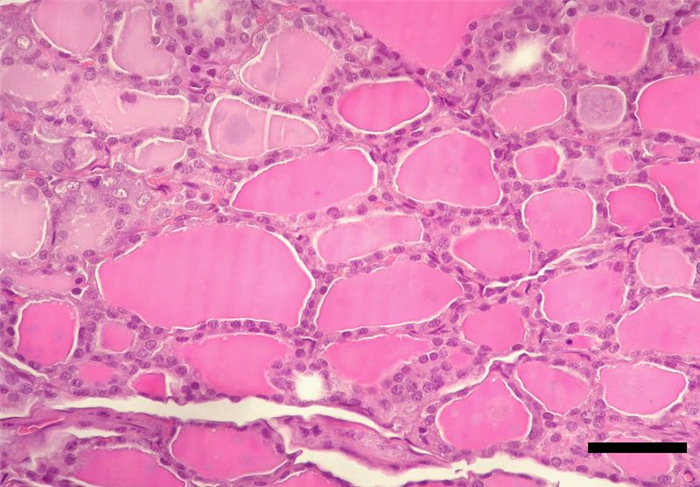
The follicle is, therefore, the structural and secretory unit of the thyroid gland, whose center is filled with a colloidal secretion (Figure 9), comprising the hormones triiodothyronine (T3) and thyroxine (T4) [4]. In between the follicles, in a parafollicular position, cells with a pale cytoplasm, and a basal nucleus can be found – parafollicular cells or C cells which produce calcitonin [4, 16, 18].
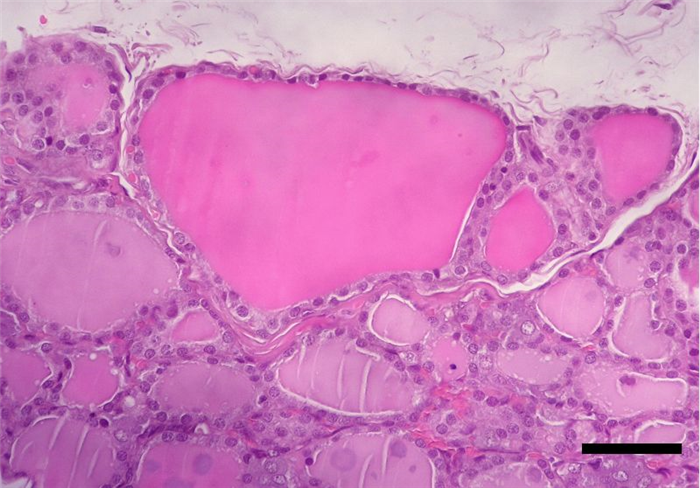
T4 and T3 have very similar effects in regulating cellular metabolism. Calcitonin assists with the regulation of calcium ion concentration in the blood. It is secreted in response to hypercalcemia, decreasing the resorption of calcium from bones and inhibiting the phosphate reabsortion from renal tubules [10] (table 3).
Due to the action of T3 and T4, thyroid is the main gland, acting in the regulation of metabolism.
Thyroid stimulation is performed by TSH, originated in the adenohypophysis, under stimulation of TRH released by hypothalamus [10].
Table 3.
Hormones produced by thyroid gland and their functions.
4. Parathyroid
Parathyroid glands are salmon-coloured and distinct from the thyroid glands. In the dog there are four parathyroid glands, one external and one internal per each thyroid gland [17, 19, 20].
External parathyroid glands are capsulated and may be found in varied positions, according to the species, which means that they can be placed between a cranial location to the thyroid gland and the entrance of the chest. In dogs, external parathyroid glands are located very close to the thyroid gland, on its cranial pole and externally to its capsule (Figure 10) [17, 19, 20].
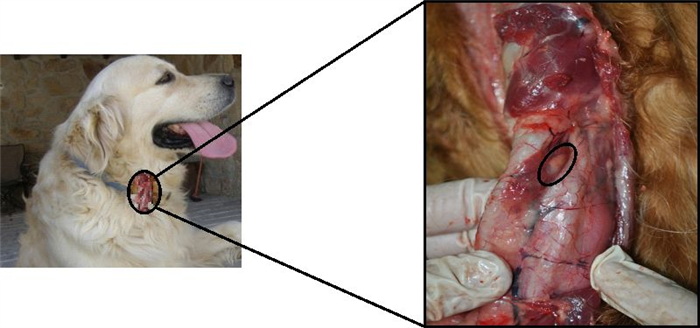
The internal parathyroids are unencapsulated glands. They may be absent in some species or may be found included within the thyroid or close to them. In the dog, internal parathyroid glands are within the thyroid capsule, in the caudal and medial aspects of the thyroid [19, 20].
The parenchyma of parathyroid glands is composed of secretory cells, named chief or principal cells, arranged in cords, clusters, chains or rosettes (Figure 11, Figure 12). In some species, such as cattle and humans, it is also composed by oxyphilic cells, organized in small clusters which have yet no known functions [4]. In dogs, this type of cells were only found in senile animals [21] and were also described in canine parathyroid adenoma [22].
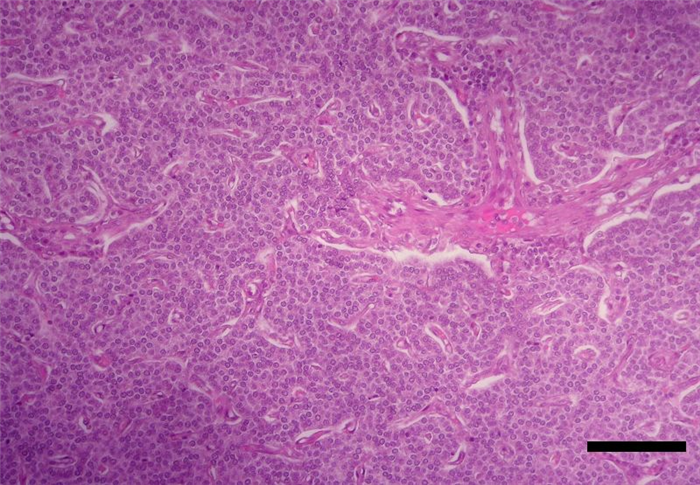
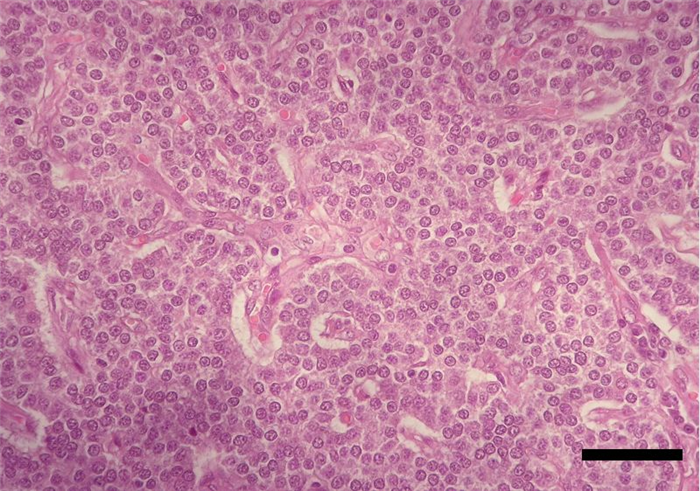
Chief cells synthesize and secrete PTH. This hormone, together with calcitonin and vitamin D, is involved in the regulation of calcium homeostasis. It is released in response to low blood calcium level [23] due to it’s ability to enhance the mobilization of calcium ions from the small intestine and from bone resorption, and also by increasing the simultaneous absorption of calcium and excretion of phosphate from distal convoluted tubules of kidneys [10] (Table 4).
Table 4.
Hormone production by parathyroid glands.
5. Pancreas
The pancreas is located in the abdominal cavity. In the dog, it is a V-shaped gland with a body and two lobes. It is acutely flexed with the apex nestling close to the cranial flexure of the duodenum. The slender right lobe runs within the mesoduodenum and the thicker but shorter left lobe extends over the caudal surface of the stomach towards the spleen, within the greater omentum (Figure 13) [4, 17].
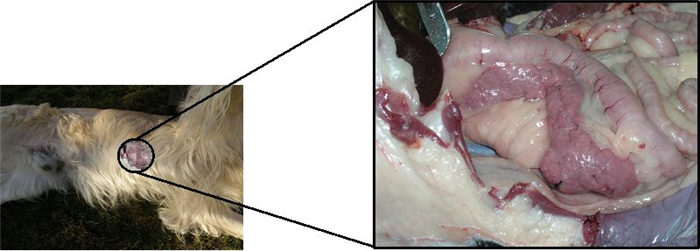
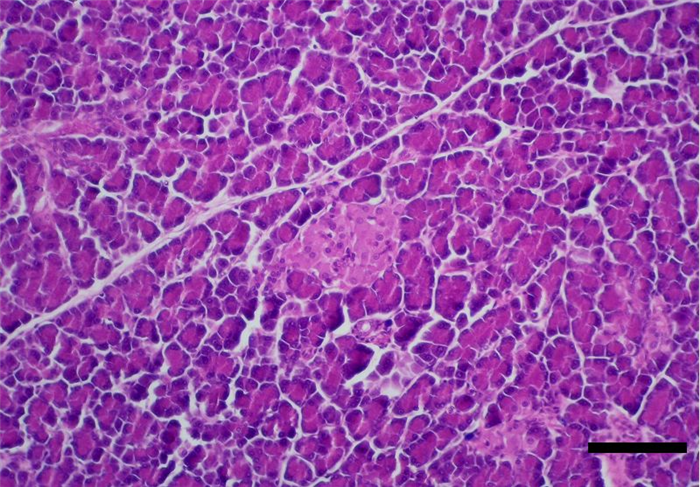
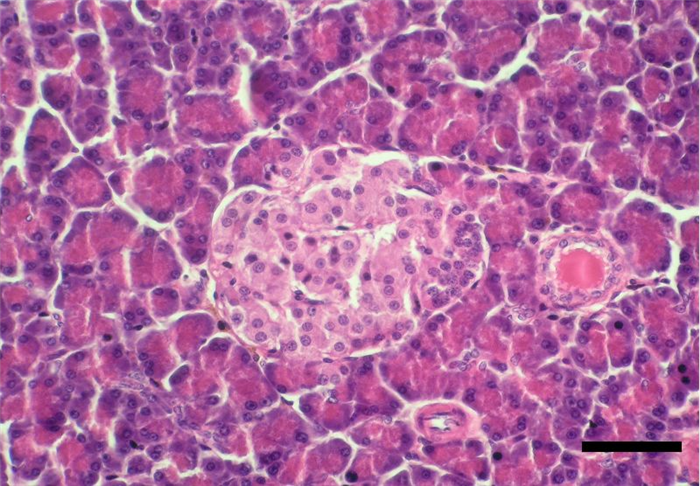
The pancreas is one of the mixed glands of the body, since it produces both exocrine (pancreatic juice, important for digestion) and endocrine secretion. The later is produced in the islets of Langerhans, which are randomly scattered in the exocrine parenchyma (Figure 14) [10, 17, 24]. Pancreatic islets are aggregates of endocrine cells required for blood glucose control and diabetes prevention after birth, consisting of polygonal shaped cells, with pale eosinophilic cytoplasm and coarse heterochromatin. Cells are arranged in cords or clusters separated by sinusoids and are subdivided in the following subtypes (Figure 15) [4, 24, 25]:
- α cells, located peripherally and representing 10-20% of islet cells, secrete glucagon, a hyperglycaemic hormone, as well as cholecystokinin, gastric inhibitory protein and ACTH-endorphin [4, 10, 24, 26].
- β cells, located in the centre of the islets, are more abundant, representing 60-80% of the islets of Langerhans, and secrete insulin, a hypoglycaemic hormone [4, 10, 24].
- δ cells, responsible for the production of somatostatin, a hormone responsible for preventing drastic variations in both insulin and glucagon [10, 24, 27]. This hormone is also capable of reducing intestinal motility and secretion of digestive juices. These cells represent about 5-40% of the islets [10, 24].
Besides the above-mentioned cell types, the endocrine pancreas also contain some minor types of cells, that represent around 5% of the total pancreatic cells. These are divided as follows:
- F cells, less abundant (2-3%), secrete pancreatic polypeptide (PP), which affects the production of some digestive enzymes and inhibits biliary and pancreatic secretion, as well as, intestinal motility [10, 24].
- D1 cells, that secrete the vasoactive intestinal peptide (VIP) [4] with important functions in the regulation of intestinal motility and intestinal transport of ions and water [10].
- EC or enterochromaffin cells, wich secrete secretin, wich stimulates bicarbonate and pancreatic enzyme secretion, and motilin that increases gastric and intestinal motility.
Insulin and glucagon are pancreatic hormones that play pivotal roles in regulating glucose homeostasis and metabolism, which have opposite effects on glycaemia as well as on the metabolism of nutrients [24, 26, 28]. In general, it can be said that:
Insulin – promotes glucose uptake by peripheral tissues, (muscle, liver and adipose tissue) and its accumulation as glycogen and fat, resulting in an anabolic effect [28] and in the removal of glucose from the blood, reducing glycaemia – hypoglycaemic effect [24];
Glucagon – promotes glucose production (catabolic effect) activating liver glycogenolysis and gluconeogenesis, releasing glucose in blood circulation, increasing glucose levels in the blood, promoting glycaemia – hyperglycaemic effect [24, 28].
So, the actions of the two hormones combined contribute to the control of blood glucose.
Glucagon secretion by α-cells is highly regulated by multiple factors, being the most important the glucose and insulin levels. Low glucose levels activate specific channels in the brain and in pancreatic α-cells to generate action potentials of sodium and calcium currents, leading to glucagon secretion. Also, somatostatin inhibits glucagon secretion by inhibiting adenylate cyclase and cAMP production [26].
Insulin has several physiologic actions that include stimulation of cellular glucose and potassium uptake [29]. Glucose-induced insulin secretion from pancreatic β-cells depends on mitochondrial activation, amongst other factors, like ATP, glutamate and others [30]. Incretin hormones, glucose-dependent insulinotropic polypeptide (GIP) and glucagon like peptide-1 (GLP-1), secreted by cells of the gastrointestinal tract in response to meal ingestion, exert important glucorregulatory effects, including the glucose-dependent potentiation of insulin secretion by pancreatic β-cells [31].
Table 5 summarizes hormones of the pancreas and their functions.
Table 5.
6. Adrenal glands
The adrenal glands are paired organs located against the roof of the abdomen near the thoracolumbar junction, in a position immediately prior to the kidneys and close to their cranial poles (Figure 16). Generally elongated, these glands are often asymmetrical and quite irregular [17].
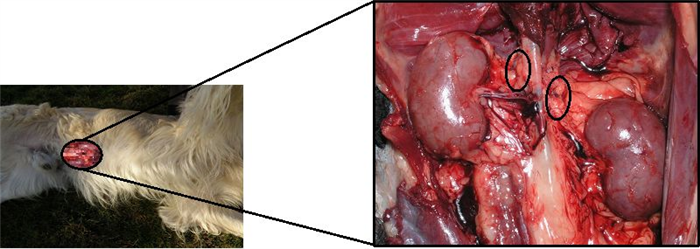
Each of these glands is divided into cortex and medulla. The cortex is yellowish and radially striated, while the medulla is more uniform and darker [17].
Both regions, cortex and medulla, correspond to areas specialized in producing different hormones [4, 32, 34]:
- The cortex consists of polyhedral secretory cells, arranged into two layered thick cords, which originate radially from the medullar zone [4, 33]. It is composed of 3 distinct zones. The outer zone is the zona glomerulosa , a thin region that in dogs and cats is composed by cells arranged in an arched or arcuate pattern, therefore also named as zona arcuata (Figure 17) [32, 35, 36].
The zona glomerulosa produces mineralocorticoids, particularly the steroid hormone aldosterone [32, 37], which is responsible for increasing sodium retention and water reabsortion in the distal tubules of the kidneys and sodium reabsorption in intestine. Aldosterone also maintains blood concentration and stimulates potassium excretion by the kidneys, thereby, indirectly regulating extracellular fluid volume. A decrease of mineralocorticoids, by loss of this zone or its functional ability, gives rise to water outlet of the blood to the tissues and may result in death, due to retention of high levels of potassium with excess loss of sodium, chloride and water [32].
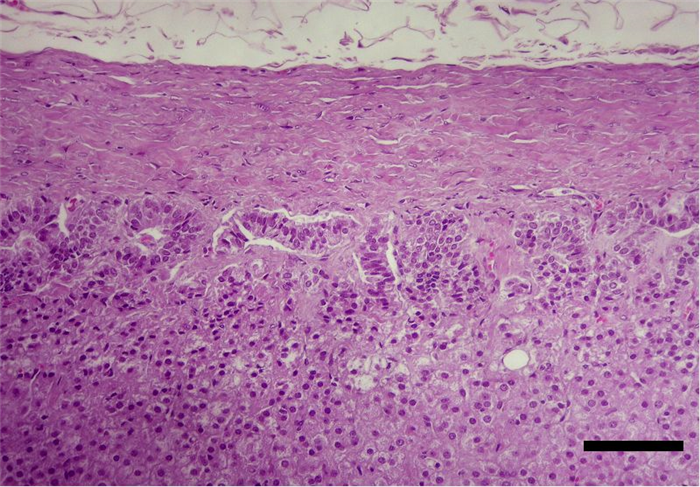
The zona fasciculata is the middle and thickest zone (corresponding to more than 70% of the cortex) and is composed of parallel columns of secretory cells, one to two cells thick, separated by prominent capillaries. The cells are cuboidal or polyhedral, containing vesicular nucleus, frequently binucleated) and foamy cytoplasm (intracellular lipid droplets) (Figure 18). This zone produces glucocorticoids [32, 33, 34, 37].
Glucocorticoids have several functions, including protein catabolism and stimulation of the hepatic gluconeogenesis from amino acids [10, 37]. In dogs, cortisol is the main glucocorticoid produced.
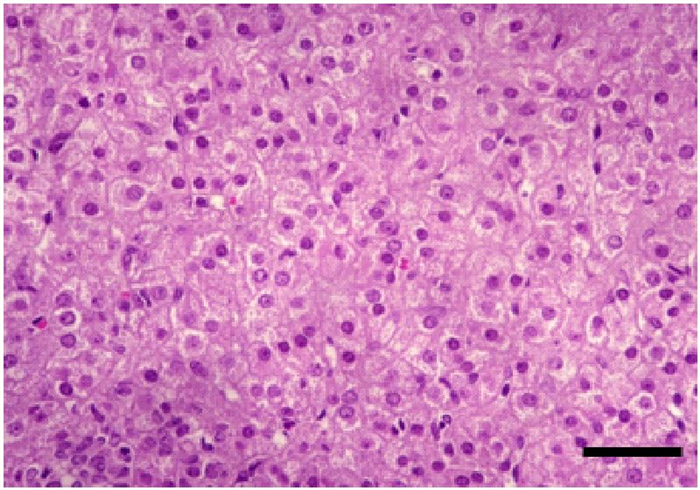
The zona reticularis is also composed of polyhedral cells, whose arrangement consists in freely anastomosing cords (Figure 19) [32]. These cells contain less lipid content but have densely granular cytoplasm for which they are called “compact cells” [34]. The zona reticularis works as a unit together with the zona fasciculata , producing androgens (androstenedione) and also glucocorticoids [34, 37].
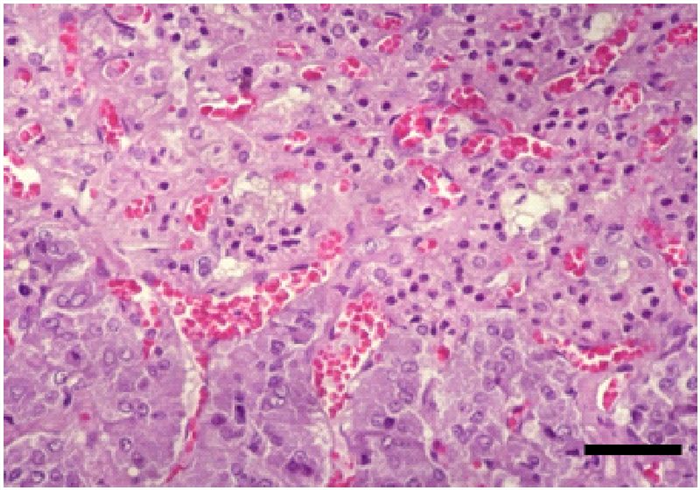
The adrenal cortex is stimulated by ACTH, produced in the adenohypophysis. In response, it produces glucocorticoids (e.g. cortisol), mineralocorticoids (e.g. aldosterone) and minor amounts of sex steroids (androgens and estrogens).
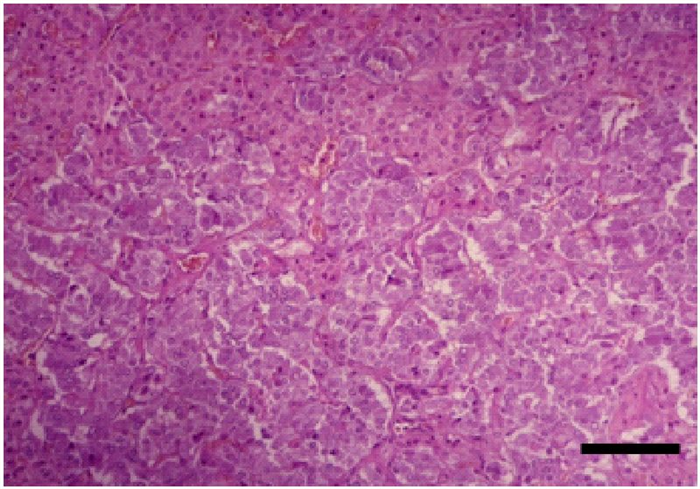
- The medulla consists of large columnar or polyhedral secretory cells, randomly distributed, with rich blood supply (Figure 20). The cells have large, vesicular nucleus, basophilic cytoplasm with fine positive chromaffin granules, due to the presence of catecholamines such as epinephrine and norepinephrine, which after exposure to oxidizing agents, such as chromate, yield a brown reaction by the formation of colored polymers [4, 31, 33].
The adrenal medulla of dogs has a heterogeneous population of cells [38]. In adults, there are 3 types of adrenal medullary cells (1): epinephrine cells (66–75%), norepinephrine cells (25–33%) and small granule-containing cells (SGC, 1-4%). The adrenal SGC cells of dogs vary from cells with a few granules and a high nucleo-cytoplasmic ratio to cells filled with many granules and a large mass of cytoplasm. Most of the chromaffin granules of these cells are small, ranging from 100 to 200 nm in diameter [39]. The medullary cells produce other peptides in addition to epinephrine and norepinephrine, such as met-enkephalin, substance P, neurotensin, neuropeptide Y, and chromogranin A. The adrenal medulla contains also presynaptic sympathetic ganglion cells, randomly scattered [4, 32].
The first step in the synthesis of epinephrine is the enzymatic conversion of tyrosine to dihydroxyphenylalanine (DOPA) by tyrosine hydroxylase. This is the rate-limiting step of hormone synthesis. DOPA is then converted to dopamine within the cytosol by decarboxylation. Dopamine can also be converted to norepinephrine by hydroxylation. Norepinephrine then leaves the granule to be converted into epinephrine in the cytosol by phenylethanolamine-N-methyltransferase (PNMT), and epinephrine re-enters the granule for storage in the cell. The activity of PNMT is induced by the high local concentration of glucocorticoids in sinusoidal blood from the adrenal cortex [10, 32]. Acute stress, hypoglycemia or other similar situations, result both in catecholamine secretion and in transsynaptic induction of catecholamine biosynthetic enzymes, including tyrosine hydroxylase. Other environmental influences, including growth factors, extracellular matrix, and a variety of hormonal signals that generate cyclic AMP, also may regulate the function of chromaffin cells [32].
In table 6, we can see a summary of some of the hormones produced by adrenal glands and their functions.
Table 6.
7. Clinical significance
Endocrine diseases, associated to altered functions of endocrine glands, are frequently seen in veterinary practice. The endocrine glands described may be targeted by several conditions summarized in table 7.
Table 7.
Pathologic conditions affecting endocrine glands.
In dogs, the most common endocrine diseases are summarized in table 8.
Table 8.
Most common endocrine diseases in dogs.
Hypothyroidism may result from destruction of the thyroid gland (primary hypothyroidism), decreased hypothalamic secretion of TRH or inadequate pituitary TSH secretion. Over 95% of canine hypothyroidism cases are primary [40, 41]. Two histologic forms of primary hypothyroidism predominate in the dog: lymphocytic thyroiditis and idiopathic atrophy. Regardless of the aetiology, the end result is the same: progressive destruction of the thyroid gland and consequent deficiency of circulating thyroid hormones [40, 41].
Hypothyroidism occurs mainly in middle aged, purebred dogs. Doberman Pinschers and Golden Retrievers are the two breeds most frequently described [42]. Clinical signs associated with hypothyroidism are varied, and behavioural changes are associated with a reduced metabolic rate, dermatological signs, cardiovascular and neuromuscular abnormalities. Obesity, in different grades is always present. Lethargy and cold intolerance are also frequently observed. Seborrhoea, bilateral and symmetrical alopecia, and pyoderma are also common dermatological symptoms. These signs can be accompanied by bradycardia, low voltage ECG complexes and weakness [40, 41, 43, 44].
Diabetes mellitus is the result of any situation that affects insulin production, insulin transport or the sensitivity of target tissues to insulin [45]. Many factors are known to contribute to the development of diabetes and its complications [46, 47]. These include genetics, diet, sedentary lifestyle, perinatal factors, age, obesity and inflammatory causes [47, 48].
Canine diabetes mellitus is generally classified as insulin dependent or non-insulin dependent based on the need for insulin treatment [40, 48]. Most diabetic dogs are thought to have a disease like human type I diabetes mellitus and are insulin dependent [40, 49, 50]. Unlike human type I diabetes mellitus, which occurs mainly in young human patients, in dogs it is more likely to occur later in life [40, 45, 48]. Dogs do not appear to progress from obesity-induced insulin resistance to type 2 diabetes mellitus, probably because pancreatic beta cells in dogs are either not sensitive to toxicity due mild hyperglycemia or lack other components of the pathophysiology of beta cell failure in type 2 diabetes mellitus [51]. Certain breeds, including Australian Terrier, Keeshounds, Alaskan Malamutes, Finnish Spitzes, Standard and Miniature Schnauzers, Miniature Poodles, and English Springer Spaniels, seem to be at increased risk to develop diabetes. Others, such as Boxers, German Shepherd dogs, Cocker Spaniels and Collies seemed to be at decreased risk [40, 52]. Typical clinical signs are polyuria, polydipsia, polyphagia and weight loss. Exercise intolerance or decreased activity, ketonic breath, recurrent infections (urinary tract, conjunctivitis), cataracts and hepatomegaly can also be present [45].
Hyperadrenocorticism can be spontaneous or iatrogenic. Spontaneous hyperadrenocorticism is associated with inappropriate secretion of ACTH by the pituitary (pituitary-dependent hyperadrenocorticism) or with a primary adrenal disorder (adrenal-dependent hyperadrenocorticism) [40, 53, 54]. Over 80% of dogs with spontaneous hyperdarenocorticism suffer from pituitary dependent hypercorticism, resulting in an over secretion of ACTH [53, 54]. This results in bilateral adrenocortical hyperplasia and excess cortisol production. The negative feedback loop between cortisol and ACTH is lost. Adrenal-dependent hyperadrenocorticism represent the remaining 20% of spontaneous hyperadrenocorticism and is generally associated with unilateral (or occasionally bilateral) adrenal tumours. This is a typical disease of middle age to old animals. Dogs showing pituitary-dependent hyperadrenocorticism exhibit a mean age of 7-9 years old, and those with adrenal-dependent hyperadrenocorticism, a mean age of 11-12 years old. There are some breeds that are most frequently associated with hyperadrenocorticism, but any breed can develop it. Poodles, Daschunds and small Terriers seem to be more predisposed to this disease. Most common clinical signs include polydipsia and polyuria, polyphagia, abdominal distension, liver enlargement, muscle weakness, lethargy, skin changes, alopecia, persistent anoestrus or testicular atrophy, calcinosis cutis and neurological signs [54, 55].
Hypoadrenocorticism is a syndrome associated to a deficient production of mineralocorticoid and/or glucocorticoid secretion by the adrenal glands. This can arise from a destruction of more than 90% of both adrenal cortices, causing inability to produce corticosteroids (Primary hypoadrenocorticism or Addison’s disease), or from a deficiency in ACTH production by the pituitary (Secondary hypoadrenocorticism) [56, 57]. Besides different pathophysiology, primary and secondary hypoadrenocorticism also exhibit different clinical signs. In dogs, primary hypoadrenocorticism is more representative. It is often associated with idiopathic adrenocortical atrophy, or with some therapeutic or surgical procedures, like mitotane treatment for hyperadrenocorticism or bilateral adrenalectomy. This condition generally affects young and middle age dogs with a median age of 4 years [57]. Great Danes, Portuguese Water Dogs, Rottweilers, Standard Poodles, West Highland White Terrier and Soft-coated Wheaten Terriers are the breeds in greater risk to develop hypoadrenocorticism [56]. Females, especially intact females, are in higher risk. Clinical signs may vary, from mild to severe, regarding the evolution of the disease. Acute hypoadrenocorticism can be life threatening and represents a medical emergency. The animal can be in a hypovolaemic shock, and generally is found in a state of collapse, or collapses when stressed. Weak pulse, bradycardia, abdominal pain, vomiting, diarrhoea, dehydration and hypothermia can also be present. Chronic presentation is more common than the acute disease. Animals with chronic hypoadrecocorticism may present anorexia, vomiting, lethargy, depression and weakness [57].
8. Conclusions
Malfunction of endocrine glands leads to severe multivariate syndromes, requiring a specialized medical approach and appropriate nursing care. For this reason, professionals engaged in clinical veterinary practice need to know animal organic structures and how they function as a whole, including the role of the endocrine system, its glands and their hormones. This systematic approach to endocrine disorders promotes not only a trained professional but a professional with technical and scientific knowledge, enabled with the exact notion of the involved etiopathogenic mechanisms and capable of making a diagnostic and therapeutical difference. This concerted attitude would certainly contribute to improve veterinary medical care to a level of excellence in the care of animal patients.
Acknowledgments
This work was supported by Portuguese Foundation for Science and Technology and Center for Studies in Education, Technologies and Health.
References
- 1. Done S., Goody P., Evans S., Stickland N., editors. Color Atlas of Veterinary Anatomy. The dog & Cat. Edinburgh: Mosby; 2004. p2.62, 2.66, 2.67, 2.79.
- 2. Ningying X., Xiaoling J. Molecular Characterization of Hypothalamo-pituitary-thyroid genes in pig (Sus scrofa). In Perez-Marin C. (ed.) A bird’s-eye view of Veterinary Medicine. Rijeka: InTech; 2012. p545-56 http://www.intechopen.com/books/a-bird-s-eye-view-of-veterinary-medicine/molecular-characterization-of-thyroid-peroxidase-gene-in-porcine-sus-scrofa- (accessed the 8 June 2012).
- 3. Morgan P., Williams L. The pars tuberalis of the pituitary: a gateway for neuroendocrine output. Reviews of Reproduction 1996;(1) 153–61.
- 4. Banks W.J., editor. Applied Veterinary Histology. St. Louis: Mosby; 1993. p 408-28.
- 5. Pankov I., Chekhranova M., Karpova S., Iatsyshina S.M., Sazina E. Molecular and genetic study of the role of hormones receptors, and enzymes in regulation of reproduction, lipid metabolism, and other human physiological functions. Vestnik Rossiiskoi Akademii Meditsinskikh Nauk 2005;(9) 6-13.
- 6. Nilsson A., Ohlsson C., Isaksson O., Lindahl A., Isgaard J. Hormonal regulation of longitudinal bone growth. European Journal of Clinical Nutrition 1994;48(S1) 158-60.
- 7. Trott J., Schennink A., Petrie W., Manjarin R., Vanklompenberg M., Hovey R. Triennial Lactation Symposium: Prolactin: The multifaceted potentiator of mammary growth and function. Journal of Animal Science 2012;90(5) 1674-86.
- 8. Perez-Marin C., Moreno M., Calero G. Clinical approach to the repeat breeder cow syndrome. In Perez-Marin C. (ed.) A bird’s-eye view of Veterinary Medicine. Rijeka: InTech; 2012. p337-62 http://www.intechopen.com/books/a-bird-s-eye-view-of-veterinary-medicine/clinical-approach-to-the-repeat-breeder-cow-syndrome (accessed the 8 June 2012).
- 9. Chauvigné F., Verdura S., Mazón M., Duncan N., Zanuy S., Gómez A., Cerda J. Follicle-Stimulating Hormone and Luteinizing Hormone Mediate the Androgenic Pathway in Leydig Cells of an Evolutionary Advanced Teleost. Biology of Reproduction 2012; doi: 10.1095/biolreprod.112.100784.
- 10. Möstl E. Endocrinología especial. In W. Engelhardt, Breves G (eds.) Fisiología Veterinaria. Zaragoza: Acribia; 2002. p526-38.
- 11. Macgregor D., Lincoln G. A physiological model of a circannual oscillator. Journal of Biological Rhythms 2008;23(3) 252-64.
- 12. Tse A., Lee A., Tse F. Ca2+ signaling and exocytosis in pituitary corticotropes. Cell Calcium 2012;51(3-4) 253-9.
- 13. Choi S.J., Pfeffer R.S. G proteins and autocrine signaling differentially regulate gonadotropin subunit expression in the pituitary gonadotrope. Journal of Biological Chemistry 2012;287(25) 21550-60.
- 14. Marsili A., Sanchez E., Singru P., Harney J., Zavacki A., Lechan R., Larsen P.R. Thyroxine-induced expression of pyroglutamyl peptidase II and inhibition of TSH release precedes suppression of TRH mRNA and requires type 2 deiodinase. Journal of Endocrinology 2011;211(1) 73-8.
- 15. Doniach I. Histopathology of the pituitary. Journal of Clinical Endocrinology & Metabolism 1985;14(4) 765-89.
- 16. Inyushkin A., Orlans H., Dyball R. Secretory cells of the supraoptic nucleus have central as well as neurohypophysial projections. Journal of Anatomy 2009;215(4) 425-34.
- 17. Dyce K.M., Sack W.O., Wensing C.J.G., editors. Veterinary Anatomy. USA:WB Saunders Company; 1996. p209-15.
- 18. Ehrhart N., Chapter 118: Thyroid. In: Slatter D.H. (ed) Textbook of small animal surgery. Philadelphia:Saunders; 2003. p1700-10.
- 19. Flanders J.A., Chapter 119: parathyroid In: Slatter D.H. (ed) Textbook of small animal surgery. Philadelphia:Saunders; 2003. p1711-22.
- 20. http://www.vsso.org/Parathyroid_Tumors.html (accessed the 30 August 2012).
- 21. Setoguti T. Electron microscopic studies of the parathyroid gland of senile dogs. American Journal of Anatomy 1977;148(1) 65-83.
- 22. Baba A.I., Câtoi C. Comparative Oncology. Bucharest: The Publishing House of the Romanian Academy; 2007. Chapter 16, Endocrine Tumors. Available from: http://www.ncbi.nlm.nih.gov/books/NBK9554/ Romanian Academy (accessed the 18 July 2012).
- 23. Pace V., Scarsella S., Perentes E. Parathyroid gland carcinoma in a Wistar rat. Veterinary Pathology 2003;40(2) 203-6.
- 24. Carrera J.A.P. Secreciones endocrinas del páncreas. In: Sacristán A.G., Montijano F.C., Palomino L.F.C., Gallego J.G., Silanes M.D.M.L., Ruiz G.S. (eds) Fisiología Veterinaria. Madrid:Mcgraw-Hill; 1995. p1074.
- 25. Kragl M., Lammert E. Calcineurin/NFATc Signaling: Role in Postnatal β Cell Development and Diabetes Mellitus. Developmental Cell 2012;23(1) 7-8.
- 26. Ali S., Drucker J. Benefits and limitations of reducing glucagon action for the treatment of type 2 diabetes. American Journal of Physiology – Endocrinology and Metabolism 2009;296 E415–21.
- 27. Aspinall V., O’Reilly M., Anatomy and Physiology. In: Lane D.R., Cooper B. (eds) Veterinary Nursing. London:Butterworth Heinemann; 2004. p41-4.
- 28. Quesada I., Tudurí E., Ripoll C., Nadal A. Physiology of the pancreatic α-cell and glucagon secretion: role in glucose homeostasis and diabetes. Journal of Endocrinology 2008;199 5-19.
- 29. Nguyen T.Q., Maalouf N.M., Sakhaee K., Moe O.W. Comparison of insulin action on glucose versus potassium uptake in humans. Clinical Journal of the American Society of Nephrology 2011;6(7) 1533-9.
- 30. Akhmedov D., De Marchi U., Wollheim C.B., Wiederkehr A. Pyruvate dehydrogenase E1α phosphorylation is induced by glucose but does not control metabolism-secretion coupling in INS-1E clonal β-cells. Biochimica et Biophysica Acta. 2012;15(10) 1815-24.
- 31. Nauck M.A. Incretin-based therapies for type 2 diabetes mellitus: properties, functions, and clinical implications. The American Journal of Medicine. 2011;124(1 Suppl) S3-18.
- 32. Rosol T.J., Yarrington J.T., Latendresse J., Capen C.C. Adrenal gland: structure, function, and mechanisms of toxicity. Toxicologic Pathology 2001;29(1) 41-8.
- 33. http://www.anatomyatlases.org/MicroscopicAnatomy/Section15/Plate15294.shtml (accessed the 8 June 2012).
- 34. Galac S., Reusch C., Kooistra H., Rijnberk A. Adrenals. In Rijnberk A., Kooistra H. (eds) Clinical Endocrinology of Dogs and Cats: An Illustrated Text. Dordrecht:Manson Publishing;2010. p93.
- 35. Engelkin L.R. Review of Veterinary Physiology. Jackson:Teton NewMedia; 2002. p519-23.
- 36. http://ocw.tufts.edu/Content/4/CourseHome/221047/221064 (accessed the 8 June 2012).
- 37. Carcagno A.R., 1995. Corteza Adrenal. In: Sacristán A.G., Montijano F.C., Palomino L.F.C., Gallego J.G., Silanes M.D.M.L., Ruiz G.S. (eds) Fisiología Veterinaria. Madrid:Mcgraw-Hill; 1995. p767-80.
- 38. Verhofstad A.A.J. Kinetics of adrenal medullary cells. Journal of Anatomy 1993;183 315-26.
- 39. Kajihara H., Akimoto T., Iijima S. On the chromaffin cells in dog adrenal medulla; with special reference to the small granule chromaffin cells (SGC Cells). Cell and Tissue Research 1978;191 1-14.
- 40. Feldman E.C., Nelson R.W., editors. Canine and Feline Endocrinology and Reproduction. St. Louis: Saunders; 2004. p86-151; 486-538; 252-357.
- 41. Panciera D.L. Chapter Fourteen: Canine hypothyroidism. In: Torrence A.G., Mooney C.T (eds.) BSAVA Manual of small animal endocrinology. Gloucester: British Small Animal Veterinary Association; 1998. p103-13.
- 42. Panciera D.L. Hypothyroidism in dogs: 66 cases (1987-1992). J Am Vet Med Assoc. 1994;204(5) 761-7.
- 43. Dixon R.M., Reid S.W.J., Mooney C.T. Epidemiological, clinical, haematological and biochemical characteristics of canine hypothyroidism. The Veterinary Record, 1999(145) 481-7.
- 44. Jaillardon L., Martin L., Nguyen P., Siliart B. Serum insulin-like growth factor type 1 concentrations in healthy dogs and dogs with spontaneous primary hypothyroidism. The Veterinary Journal 2011:190 e95-9.
- 45. Graham P.A. Chapter Twelve: Canine diabetes mellitus. In: Torrence A.G. and Mooney C.T (eds) BSAVA Manual of small animal endocrinology. 2 nd edition, 1998. p83-96.
- 46. Mega C., Teixeira de Lemos E., Vala H., Fernandes R., Oliveira J., Mascarenhas-Melo F., Teixeira F., Reis F. Diabetic nephropathy amelioration by a low-dose sitagliptin in an animal model of type 2 diabetes (Zucker Diabetic Fatty rat). Experimental Diabetes Research 2011; doi:10.1155/2011/162092.
- 47. King G.L. The role of inflammatory cytokines in diabetes and its complications. J Periodontol 2008;79(8)Suppl 1527-34.
- 48. Guptill L., Glickman L., Glickman N. Time Trends and Risk Factors for Diabetes Mellitus in Dogs: Analysis of Veterinary Medical Data Base Records (1970–1999). The Veterinary Journal 2003;165 240-7.
- 49. Bennet N. Monitoring techniques for diabetes mellitus in the dog and the cat. Clinical Techniques in Small Animal Practice, 2002;17(2) 65-9.
- 50. Declue A.E., Nickell J., Chang C.H., Honaker A. J. Upregulation of proinflammatory cytokine production in response to bacterial pathogen-associated molecular patterns in dogs with diabetes mellitus undergoing insulin therapy. Journal of Diabetes Science and Technology. 2012;6(3) 496-502.
- 51. Verkest K.R., Rand J.S., Fleeman L.M., Morton J.M. Spontaneously obese dogs exhibit greater postprandial glucose, triglyceride, and insulin concentrations than lean dogs. Domest Anim Endocrinol. 2012;42(2) 103-12.
- 52. Hoenig M. Comparative aspects of diabetes mellitus in dogs and cats. Molecular and Cellular Endocrinology 2002;197 221-9.
- 53. Peterson M.E. Diagnosis of Hyperadrenocorticism in Dogs. Clin Tech Small Anim Pract 2007;22 2-11.
- 54. Herrtage M.E.Chapter Ten. Canine Hyperadrenocorticism. In: Torrence A.G., Mooney C.T (eds) BSAVA Manual of small animal endocrinology. Gloucester: British Small Animal Veterinary Association; 1998. p55-73.
- 55. Grecco D. Hyperadrenocorticism Associated with Sex Steroid Excess. Clin Tech Small Anim Pract 2007;22(1) 12-7.
- 56. Herrtage M.E. Chapter Eleven: Hypoadrenocorticism. In: Torrence A.G. and Mooney C.T (eds) BSAVA Manual of small animal endocrinology. Gloucester: British Small Animal Veterinary Association; 1998. p75-82.
- 57. Grecco D. Hypoadrenocorticism in Small Animals. Clin Tech Small Anim Pract 2007;22(1) 32-5.