Endocrine System
Along with the nervous system, the endocrine system coordinates the activities of cells in different organs and tissues to control physiological parameters or generate system-wide responses to environmental changes. Information in the endocrine system is transmitted through several small molecules, peptides and proteins that function as hormones. These hormones are released by cells in one part of the body, enter the circulatory system to distribute throughout the body and bind specific receptors in other cells. Hormone receptors in the endocrine system often trigger changes in gene expression to alter the activities of key biochemical or physiological pathways. The changes in these pathways are sufficient to regulate parameters such as serum calcium or glucose or respond to external stimuli such as stress.
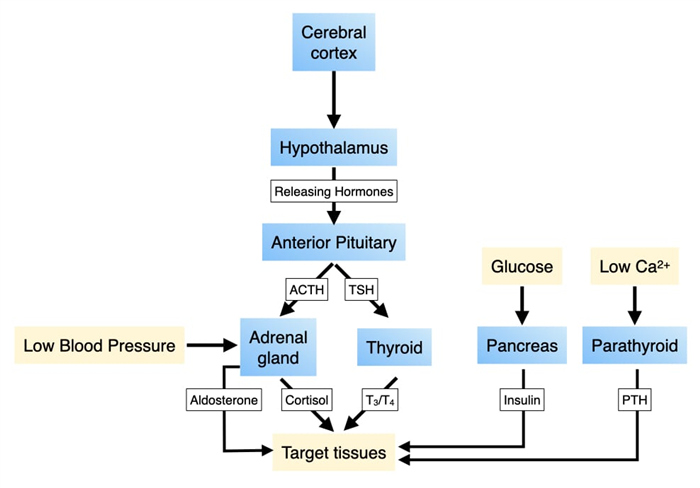
The production of hormones in the endocrine system can be regulated by the concentration of certain molecules or ions (e.g. glucose, calcium) or by the nervous system which allows for integration of external stimuli or input from higher parts of the brain. The latter pathway involves the pituitary and hypothalamus. The hypothalamus is a component of the central nervous system and regulates the release of hormones from cells in the pituitary. Some hormones produced by the pituitary directly alter the behavior of specific cells in tissues to generate physiological changes, whereas other pituitary hormones trigger cells in other organs to release hormones which change cell behavior.
Pituitary Gland
The pituitary gland is the central organ of the endocrine system as it provides an interface between the nervous system and cells in several different organs and tissues. Cells in the pituitary produce many different hormones. Some regulate the biochemical and physiological activity of other cells while others stimulate cells in other parts of the body to release their own hormones. In both cases, the hormones released from the pituitary change key physiological parameters and/or alter system-wide behavior. Neurons in central nervous system control the release of hormones from the pituitary which provides an access point for environmental stimuli to alter physiological parameters in the body.
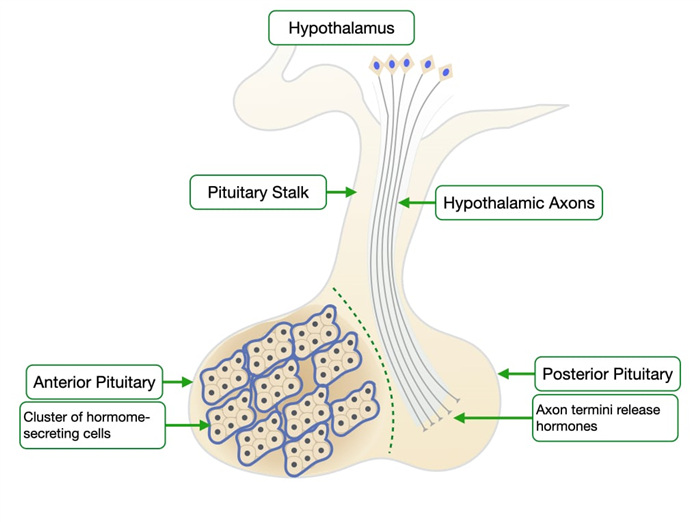
The pituitary gland resides just outside the central nervous systems and comprises two anatomical and functional structures: anterior and posterior. The anterior pituitary or adenohypophysis is a collection of cells that secrete hormones to control the activities of cells in other endocrine organs. Some of these hormones control the production of hormones in other organs while others have direct effects on basic biological properties. The posterior pituitary contains the axons of neurons whose cell bodies reside in the hypothalamus which is part of the central nervous system.
Cells in the anterior pituitary produce these hormones:
- Growth Hormone (GH) – stimulates cells to produce insulin-like growth factor 1 (IGF-1) which stimulate growth of muscle and bone.
- Adrenocorticotrophic Hormone (ACTH) – stimulates cells in the adrenal gland to produce corticosteroids
- Thyroid-Stimulating Hormone (TSH) – stimulates cells in the thyroid gland to produce the hormones T3 and T4
- Prolactin – initiates and maintains milk production in mammary glands
- Leutenizing Hormone (LH) – stimulates Leydig cells to make testosterone (will be discussed in Across the Lifespan)
- Follicle-Stimulating Hormone (FSH) – stimulates ovarian follicular cells to produce estrogens and progesterins and Sertoli cells to initiate spermatogenesis (will be discussed in Across the Lifespan)
Each of these hormones is produced by a different cell type:
- Somatotrophs produce growth hormone and make up about 50% of the anterior pituitary.
- Mammotrophs produce prolactin and compose about 20% of the anterior pituitary
- Corticotrophs produce ACTH and compose about 20% of the anterior pituitary
- Thyrotrophs produce TSH and make up 5% of the anterior pituitary
- Gonadotrophs produce FSH and LH and make up 5% of the anterior pituitary
The posterior pituitary or neurohypophysis contains the axons of neurons whose cell bodies reside in the hypothalamus which is part of the nervous system. The neurons produce and release the following hormones:
- Oxytocin – induces uterine contraction and ejection of milk from the mammary gland
- Vasopressin – induces cells in the collecting to increase absorption of water
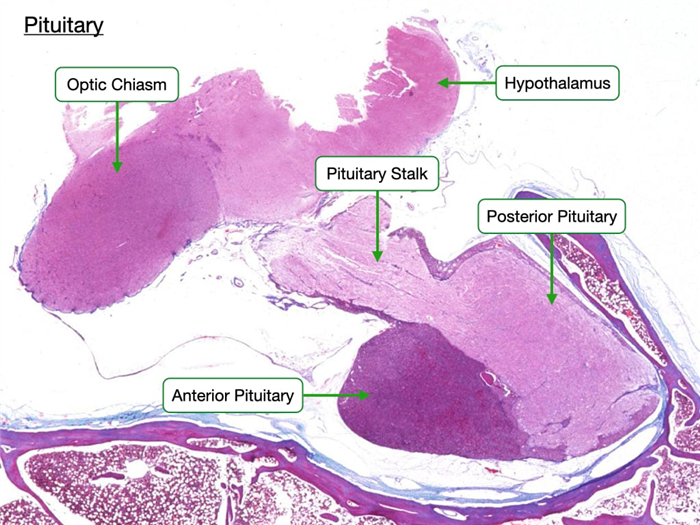
This slide shows a section of the human pituitary stained by H&E. The anterior and posterior pituitary appear strikingly different. The anterior pituitary stains much darker because it contains many more cells than the posterior pituitary. In contrast, the posterior pituitary contains mostly axons of neurons whose cell bodies are in the hypothalamus along with a few support cells. Note that the pituitary is connected to the hypothalamus by the pituitary stalk. The stalk contains axons of neurons of the posterior pituitary and also a unique capillary bed that will allow the hypothalamus to regulate the production of hormones in the anterior pituitary.
Anterior Pituitary
In H&E-stained samples of the anterior pituitary one can distinguish three types of cells: acidophils, basophils and chromophobes.
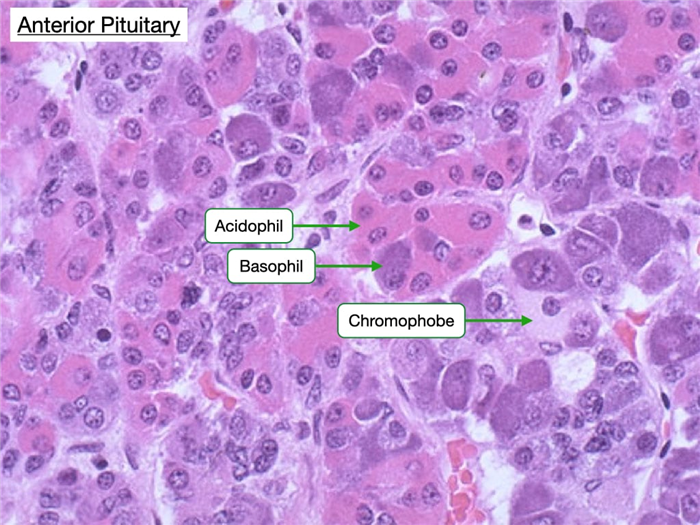
Acidophils are more eosin-stained and secrete the protein hormones growth hormone and prolactin.
Basophils appear basophilic and secrete glycoprotein hormones such as adrenocorticotrophic hormone (ACTH), thyroid-stimulating hormone (TSH), follicle-stimulating hormone (FSH), and leutenizing hormone (LH).
Chromophobes have clear nuclei and scant cytoplasm. They may be cells that are non-secretory or exhibit minimal hormone storage.
Note how all three types of cells reside in clusters and that in a single field one can usually see all three types.
One can distinguish individual cell types in the anterior pituitary by electron microscopy or immunostains.
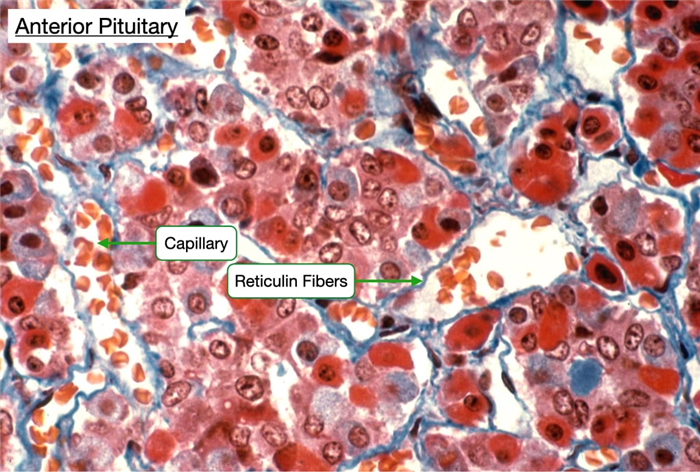
Staining the anterior pituitary for reticulin fibers reveals how the fibers organize the cells into clusters. Acidophils and basophils appear different in this type of stain. The anterior pituitary contains are large number of capillaries which facilitate delivery of releasing hormones from the hypothalamus (see below) and entry of secreted hormones into the circulatory system.
Regulation of Hormone Release in the Anterior Pituitary
The release of hormones by cells in the anterior pituitary is directly regulated by hormones produced by neurons in the hypothalamus. These hormones are termed releasing hormones because they stimulate cells in the anterior pituitary to release their hormones. These are the major releasing hormones:
- Growth Hormone Releasing Hormone (GHRH) – stimulates release of growth hormone
- Corticotropin Releasing Hormone (CRH) – stimulates release of ACTH
- Thyrotropin Releasing Hormone (TRH) – stimulates release of TSH
- Gonadotropin Releasing Hormone (GnRH) – stimulates release of FSH and LH
A unique vascular system, called the hypophyseal portal system, delivers the releasing hormones from the hypothalamus to the anterior pituitary. Cells in the hypothalamus secrete releasing hormone into tissue that is perfused by a capillary plexus. These capillaries coalesce into portal veins that deliver blood to a second capillary plexus in the anterior pituitary. The endothelium of the capillaries is fenestrated to allow for less restrictive diffusion of hormones between blood and the surrounding cells.
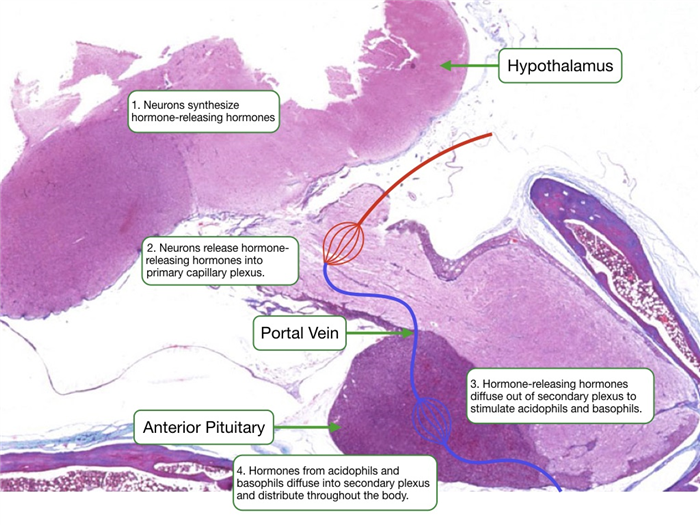
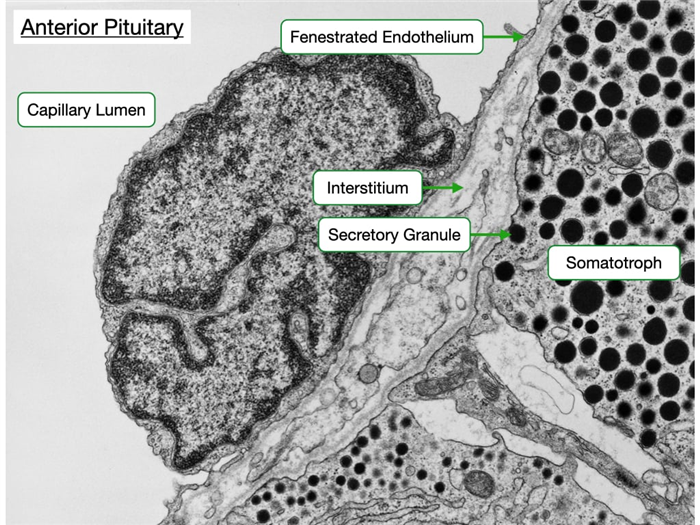
Each type of hormone-producing cell in the anterior pituitary expresses a G-protein coupled receptor that binds a specific releasing hormone from the hypothalamus. Upon binding the releasing hormone, the receptor stimulates a signaling pathway that increases cytosolic calcium and the release of hormone. Cells in the anterior pituitary store hormone in secretory granules. Increases in cytosolic calcium driven trigger the fusion of granules with the cell membrane to release the hormone into the interstitial space.
From the interstitium, the hormone diffuses across a fenestrated endothelium to enter the circulatory system. The electron micrograph below reveals the proximity of the hormone-producing cells to the fenestrated capillary. Also observe that some of the secretory granules appear docked at the cell membrane.
Posterior Pituitary
The posterior pituitary is also known as the neurohypophysis and is derived from the hypothalamus. It is mostly composed of unmyelinated axonal processes and terminals of neurons whose cell bodies reside in the hypothalamus. The neurons produce and release the hormones oxytocin and vasopressin. The posterior pituitary has a separate vasculature system from the anterior pituitary that carries away its products.

The posterior pituitary appears more lightly stained compared to the anterior pituitary due to fewer cells in the posterior pituitary. The cell bodies of the neurons whose axons release hormones in the posterior pituitary are located in the hypothalamus, so most of the nuclei visible in the posterior pituitary belong to supporting cells known as pituicytes. Pituicytes are the glial or Schwann cells of the pituitary gland. The posterior pituitary also has characteristic Herring bodies, which are focal axonal swellings packed with secretory granules.
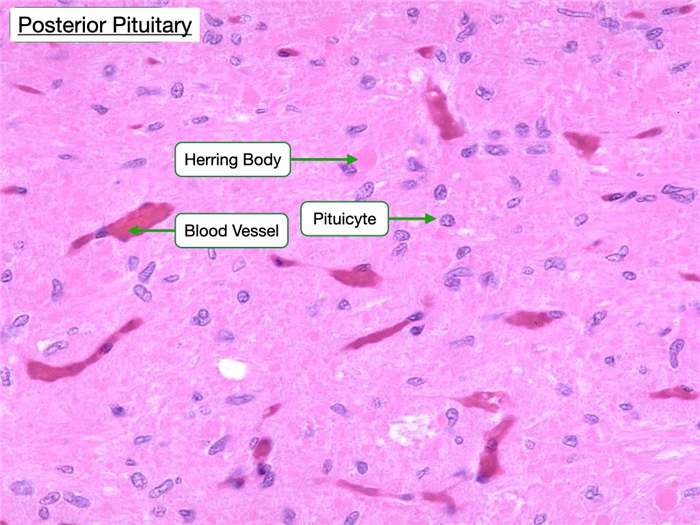
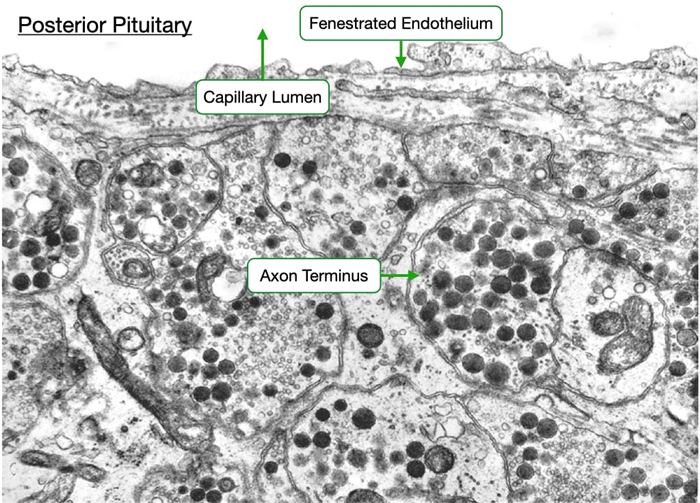
The electron micrograph below of the posterior pituitary reveals the proximity of the axon termini of the hypothalamic neurons with the vasculature. Note the endothelium of the adjacent capillary is fenestrated to allow for easier passage of oxytocin and vasopressin into the circulatory system.
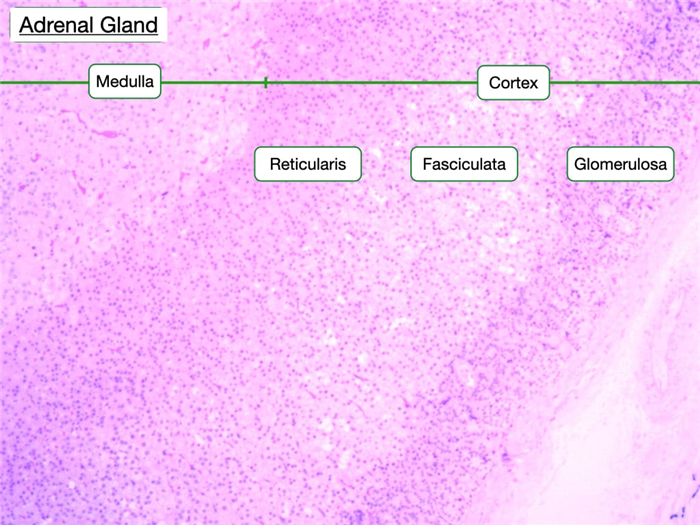
Adrenal Gland
The adrenal gland has two distinct parts, the cortex and medulla, which differ in structure, function, and embryological origin. The cortex secretes hormones produced from cholesterol and can be functionally and histologically divided into three zones: zona glomerulosa (mineralocorticoids), zona fasciculata (glucocorticoids) and zona reticularis (sex hormones). The medulla primarily secretes catecholamines, including adrenaline and noradrenaline. The three cortical zones can be distinguished on the basis of differences in the arrangement and cytology of the cells.
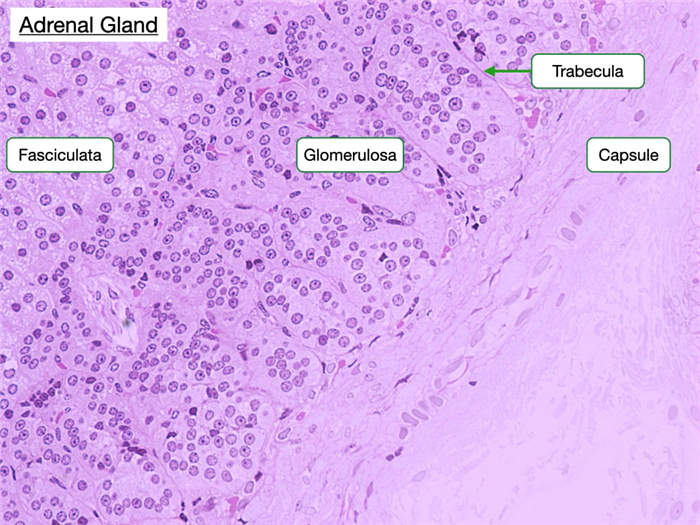
Zona Glomerulosa
The zona glomerulosa is located just beneath the capsule of the adrenal gland and contains cells arranged in clusters separated by trabeculae that are continuous with the capsule. The capsule is a connective tissue layer with collagen fibers and blood vessels. Capillaries branch from these blood vessels and extend into the trabeculae. The trabeculae organize cells into ovoid clusters. The cells of the glomerulosa appear pale-staining and have distinct round nuclei and a higher nuclear to cytoplasmic ratio than the cells of the adjacent zona fasciculata.
Cells in the glomerulosa produce mineralocorticoid hormones, such as aldosterone, from cholesterol. Increased serum potassium and decreased blood flow into nephrons stimulates the production of aldosterone. Recall that cells in the juxtaglomerular apparatus in nephrons release renin into the afferent arterioles when blood pressure is low. Renin initiates an enzymatic cascade that generates angiotensin II. Cells in the glomerulosa contain a G-protein coupled receptor to angiotensin II that activates a singling pathways that increase aldosterone production, cell growth and proliferation. Aldosterone binds mineralocorticoid receptors in cells of the distal convoluted tubule (DCT) and collecting ducts simulating their expression of Na-K pumps in the basolateral membrane and Na+ channels (e.g. ENaC) in the apical membrane. Consequently, cells in the DCT and collecting ducts can reabsorb more sodium and water to increase blood volume.
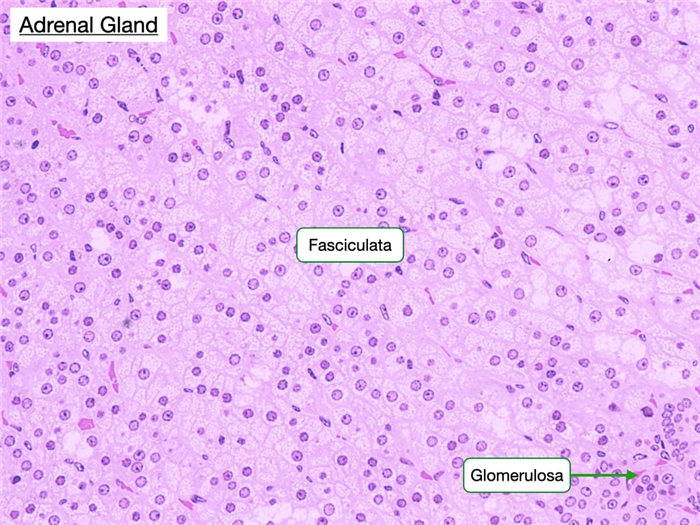
Zona Fasciculata
The zona fasciculata comprises the thick middle layer of the cortex. Its cells are extensively vacuolated because of the presence of lipid droplets. The cells of this region are organized into parallel cords separated by straight capillaries and synthesize glucocorticoids, such as cortisol, from cholesterol. Adrenocorticotrophic hormone (ACTH) produced by cells in the anterior pituitary stimulates cells in the fasciculata to increase production of cortisol.
Cortisol affects many physiological systems. It increases serum glucose levels by stimulating gluconeogenesis in the liver and inhibiting uptake of glucose in peripheral tissues, such as muscle and adipose tissue, by reducing the concentration GLUT4 channels in their cell membranes. Cortisol promotes breakdown of protein to amino acids in muscle and lipolysis in adipose cells to provide the liver with macromolecules to convert to glucose. Cortisol is also a powerful immunosuppressant and anti-inflammatory agent.
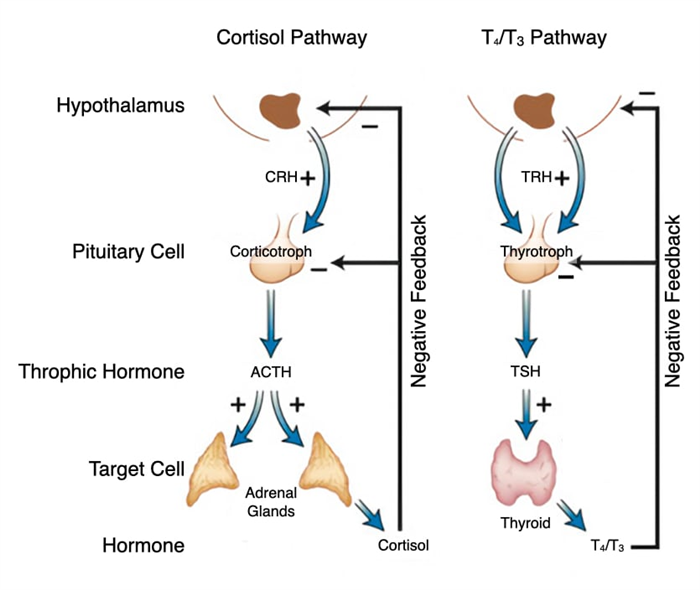
Stress, both internal and environmental, stimulates production of cortisol. Stress triggers neurons in the hypothalamus to release corticotropin-releasing hormone (CRH) which flows through the hypophyseal portal system into the pituitary. In the pituitary, CRH stimulates corticotrophs to release ACTH which flows through the bloodstream to the adrenal gland and stimulates cells in the fasciculata to release cortisol. The relationship between hypothalamus, pituitary and adrenal gland is referred to as the hypothalamic-pituitary-adrenal axis.
Cortisol also regulates its own production through negative feedback on the hypothalamus and anterior pituitary. Cortisol decreases synthesis of corticotropin-releasing hormone in the hypothalamus and reduces synthesis of the CRH receptor so that corticotrophs don’t respond to CRH. Cortisol also decreases synthesis of ACTH in corticotrophs. Thus, by reducing production of ACTH, cortisol inhibits its own release.
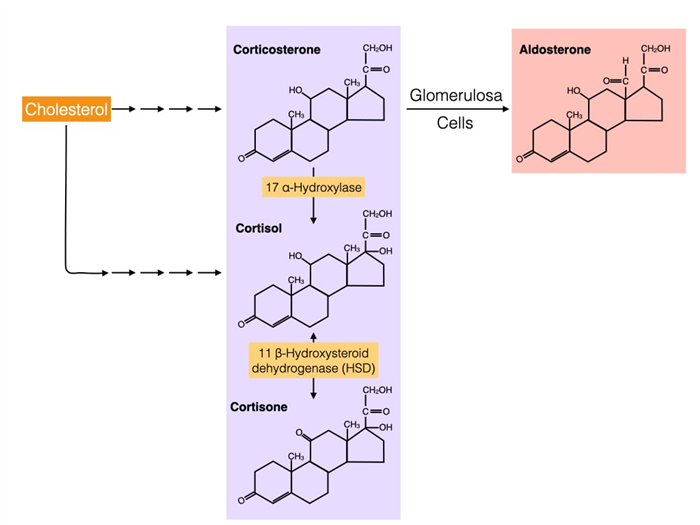
Cross-talk between corticosteroids and aldosterone
Structurally, cortisol and aldosterone are very similar and are produced by the same pathway that starts with cholesterol. The pathways diverge at corticosterone which is converted to aldosterone by cells in the glomerulosa or to cortisol by cells in the fasciculata. Cortisol can be converted to cortisone by the enzyme 11 beta-hydroxysteroid dehydrogenase (11-β-HSD). Cortisone binds weakly to glucocorticoid receptors and the conversion of cortisol to cortisone is the first step in reducing cortisol activity. 11-β-HSD can also catalyze the reverse reaction where cortisone is converted into cortisol which is why cortisone has therapeutic effects.
An isozyme of 11-β-HSD, called 11-β-HSD2, has a critical role in the kidney where it converts cortisol to cortisone in cells that line part of the nephron. Recall that the mineralocorticoid receptor binds aldosterone and triggers an increase in sodium reabsorption. The mineralocorticoid receptor also bind cortisol with high affinity and cortisol is usually present at higher concentrations in the body compared to aldosterone. However, under normal conditions cortisol does not increase sodium reabsorption in the kidney because cells of the nephron express 11-β-HSD2 which converts cortisol to cortisone. Cortisone weakly binds the mineralocoirtcoid receptor.
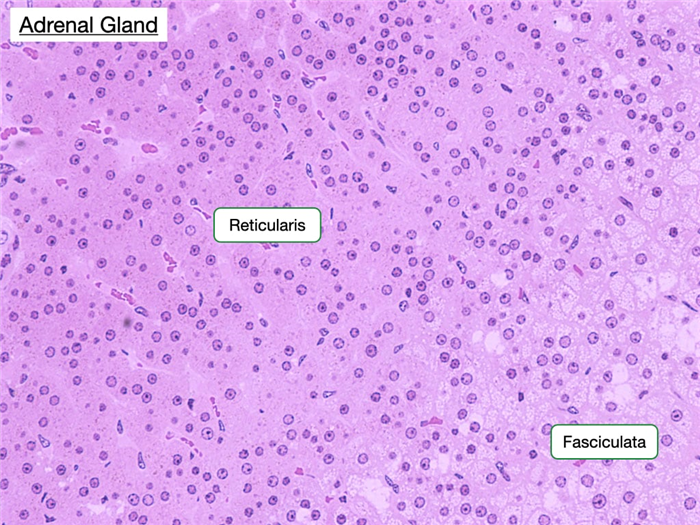
Zona Reticularis
The zona reticularis is the innermost layer of the adrenal cortex. The border between it and the zona fasciculata is less distinct than that between the previous two zones. Cells in the zona reticularis stain deeply and are less vacuolated. This region produces androgens, which supplement sex hormones produced by the gonads.
Adrenal Medulla
The adrenal medulla is the innermost portion of the gland and shares an embryological origin with the sympathetic nervous system. Its cells possess abundant cytoplasmic granules that contain stored peptide hormones and catecholamines (epinephrine and norepinephrine). These cells are frequently called chromaffin cells because they can be stained with chromium salts. Preganglionic sympathetic fibers traverse the adrenal cortex and synapse directly on chromaffin cells. During a sympathetic response, often caused by external stress, the preganglionic neurons release acetylcholine which simulates in chromaffin cells the fusion of granules with the cell membrane to release catecholamines. Epinephrine has myriad physiological effects on the body including increasing heart and respiratory rate, serum glucose concentration and blood flow to skeletal muscle. Norepinephrine has more specific effects on heart rate. Note the abundant vasculature of the medulla which facilitates entry of catecholamines into the circulatory system.
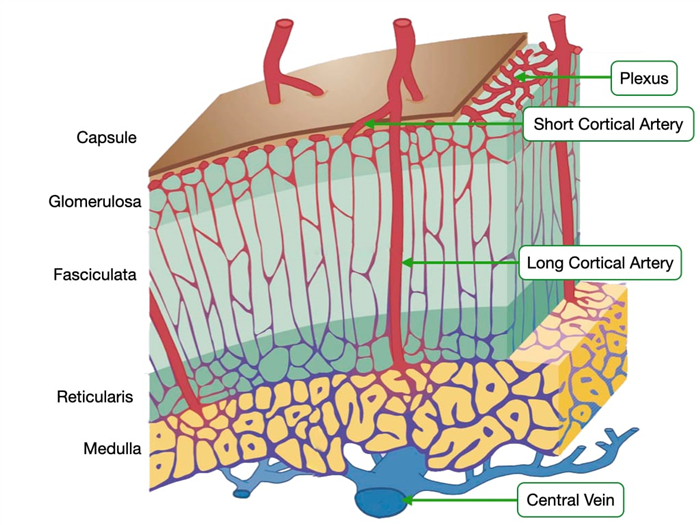
Adrenal Gland – Blood Flow
The adrenal glands receive a relatively large amount of blood for their weight. Several branches of the suprarenal arteries deliver blood to the adrenal glands. The arteries enter at the capsule and form a plexus beneath the capsule of the gland. Blood from these smaller arteries flows through two different pathways to reach the medulla where blood exits through the central vein.
In one pathway, arteries in the plexus give rise to an anastomosing network of capillary sinusoids. These sinusoids descend down through the three layers of cortex and into the medulla. This pattern of blood flow exposes cells in the medulla to high concentrations of glucocorticoids and mineralocorticoids that were synthesized in the cortex. Glucocorticoids stimulate the conversion of norepinephrine into epinephrine and can modulate the body’s response to stress.
In the second pathway, long cortical arteries course through the cortex and form a large capillary network around the secretory cells of the medulla and then drain into the central vein of the medulla. Because the medulla has capillaries from both pathways, it is more highly vascularized compared to the other regions of the adrenal gland.
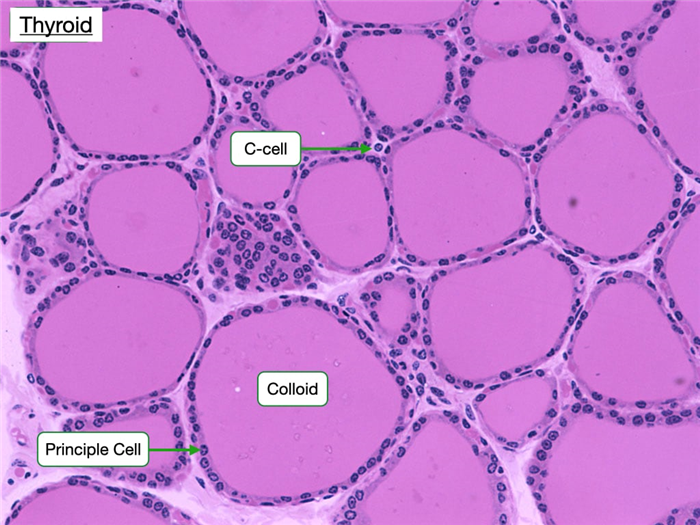
Thyroid
The thyroid is located in the neck and is surrounded by a collagenous capsule continuous with the middle cervical fascia. It is unique because it stores large amounts of inactive hormone within extracellular compartments, whereas in most glands cells store small amounts of hormone intracellularly. The thyroid consists of structural units called follicles, which are composed of secretory epithelial cells, called principal cells, that are adjoined by junctional complexes and surrounded by a basement membrane and reticular connective tissue. Follicles vary in size, but each displays a central lumen containing colloid. Colloid consists of the glycoprotein thyroglobulin, which is secreted by the principal cells and serves as a precursor to thyroid hormone. The apical surface of principle cells faces colloid.
The height of the principal cells varies according to their level of secretory activity. When unstimulated as in hypothyroidism, the cells are squamous or cuboidal, whereas in overstimulation or hyperthyroidism, they are columnar.
The thyroid contains another cell that appears to function in regulating serum calcium levels and bone density. Parafollicular cells (C-cells) lie adjacent to the principal cells but and do not extend into the follicular lumen. They secrete the hormone calcitonin, which inhibits the absorptive activity in osteoclasts. High serum calcium concentrations stimulate C-cells to release calcitonin. When serum calcium levels are below normal, calcitonin release is inhibited.
The production of thyroid hormone follows several well-defined steps. First, principle cells synthesize and secrete a large protein called thyroglobulin into colloid. Principle cells also transport iodide anion (I – ) from their basal surface into colloid. Vectorial transport of I – depends upon the Na/I co-transporter along the basal surface of principle cells and the Cl-I exchanger on the apical surface.
The second step in thyroid hormone production is to iodinate thyroglobulin on tyrosine residues. An enzyme on the apical surface of principle cells called thyroid peroxidase (TPO) converts I – to I o (iodine) which readily reacts with tyrosine. TPO also catalyzes a reaction that couples two iodinated tyrosine within a thyroglobulin molecule. The coupled, iodinated tyrosine will eventually become active thyroid hormone.
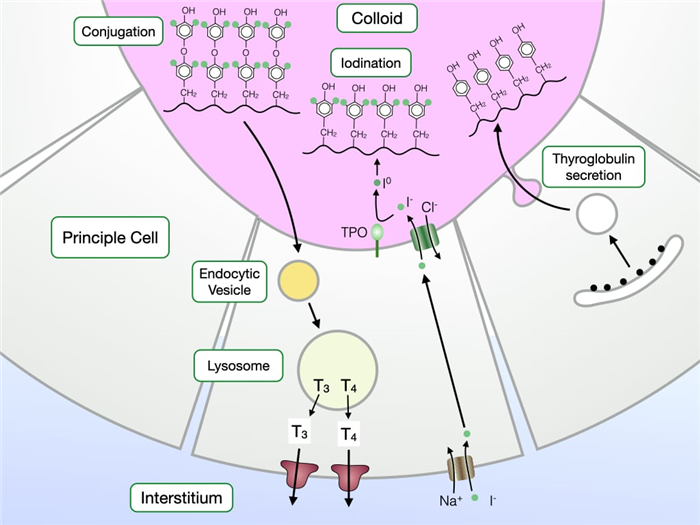
The final steps of thyroid hormone production release coupled, iodinated tyrosine from thyroglobulin. First, principle cells take up thyroglobulin from colloid via endocytosis. Thyroglobulin is delivered to the lysosome where enzymes hydrolyze thyroglobulin to release coupled, iodinated tyrosine which is eventually secreted at the basal surface where it enters the circulatory system.
Principle cells produce two forms thyroid hormone. T4 or tetraiodothyronine contains two tyrosines each with two iodines. T3 or triiodothyronine contains two tyrosines, one with two iodines and one with one iodine. T3 is more biologically active than T4 and 90% of the thyroid hormone produced by principle cells is T4. Peripheral tissues contain a deiodinase that converts T4 to T3.
T3 maintains a basal metabolic rate in cells but can also increase the metabolic rate in response to stressors like cold exposure. Cold causes neurons in the hypothalamus to release thyrotropin-releasing hormone (TRH) which travels to the anterior pituitary through the hypophyseal portal system. TRH stimulates thyrotrophs to release thyroid stimulating hormone. Thyroid-stimulating hormone (TSH) stimulates production of T4 and T3 by increasing the rates of several steps in the synthesis of T3 and T4. TSH increases vectorial transport of iodine, iodination and conjugation of tyrosine in thyroglobulin, endocytosis and proteolysis of thyroglobulin into T4 and T3, and secretion of T4 and T3.
T3 stimulates the metabolic rate in cells and thermogenesis in muscle and brown fat by increasing expression of uncoupling proteins in mitochondria. Recall that uncoupling proteins dissipate the proton gradient across the inner membrane of mitochondria so that heat instead of ATP is generated.
T4 and T3 also exert negative feedback on their own production. First, they inhibit the synthesis of TRH in the hypothalamus. Second, they reduce the number of TRH receptors on thyrotrophs in the anterior pituitary which reduces the synthesis and release of TSH.

Parathyroid
The parathyroid glands are closely associated with the thyroid and consist of closely packed groups of two cell types.
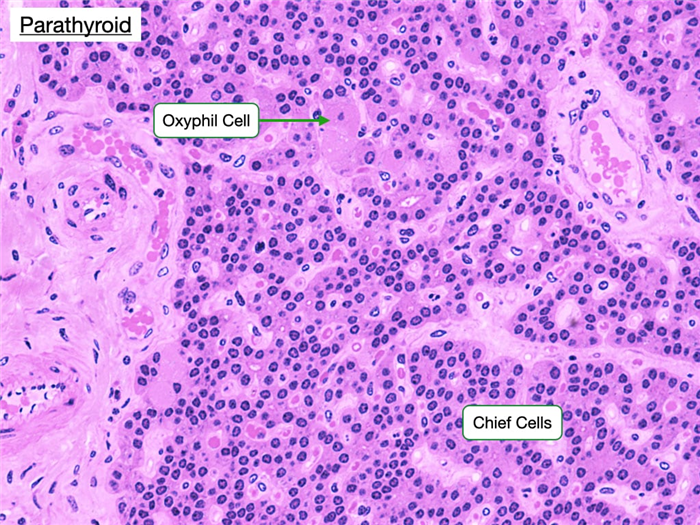
Chief (principal) cells, which have prominent central nuclei surrounded by pale cytoplasm. Chief cells produce parathyroid hormone (PTH), which is the most important regulator of calcium metabolism in humans. When serum calcium levels fall, chief cells release PTH which indirectly stimulates the production of osteoclasts in bone. Osteoblasts contain receptors for PTH, whereas osteoclasts do not. PTH stimulates osteoblasts to produce proteins such as RANKL that stimulate the development of osteoclasts, degradation of bone and release of calcium.
Chief cells contain a calcium-sensing receptor which is a G-protein coupled receptor. When serum calcium levels are normal, the receptor is more likely bound to calcium and stimulates a pathway that increases intracellular calcium. In contrast to most cells, high intracellular calcium concentration inhibits the fusion of secretory granules, which contain PTH, with the cell membrane. Decreases in serum calcium deactivate the pathway leading to a fall in cytosolic calcium and fusion between secretory granules and the cell membrane to release PTH.
Oxyphilic cells are also found in the parathyroid. They are large but fewer in number than chief cells and have small, dark nuclei and an acidophilic cytoplasm with many mitochondria. The function of these cells is unknown, but they increase in abundance as a person ages.
Endocrine Pancreas
The endocrine portion of the pancreas is comprised of the islets of Langerhans. The islets of Langerhans appear as distinct islands in a sea of pancreatic acinar cells, and constitute just a small percentage (2%) of pancreatic tissue. Regardless of the stain used, the islets can be identified because of their distinct geometric cord pattern and high vascularity when compared to the acini. The islets contain three important cell types.
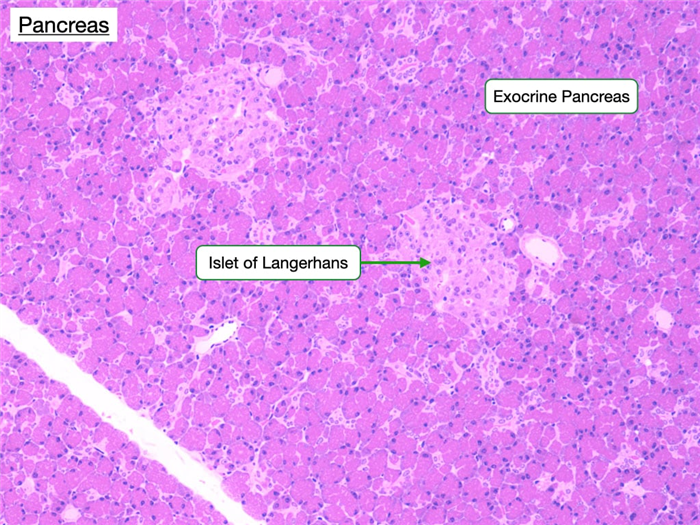
- α cells produce glucagon, which increases the plasma glucose concentration.
- β cells produce insulin, which decreases plasma glucose by promoting uptake by cells in the liver, skeletal muscle, and adipose tissue.
- δ cells produce somatostain, which has broad effects on gastrointestinal function and inhibits insulin and glucagon secretion. D-cells are scattered throughout the islets.
Beta cells secrete insulin in response to an increase in concentration of glucose in serum. Glucose enters beta cells through the GLUT2 transporter. Beta cells convert metabolize glucose through glycolysis and the TCA cycle to generate ATP. As cytosolic ATP concentration rises, ATP-sensitive potassium channels in the cell membrane close, depolarizing the membrane. Increase in membrane potential (more positive) triggers voltage-gated calcium channels to open. The increase in cytosolic calcium allows secretory granules with insulin to fuse with the cell membrane.