Posterior Pituitary Endocrine Function
The pituitary gland is a small endocrine organ found in the brain. It consists of two lobes – the anterior and posterior pituitary. The lobes are embryologically and histologically different and effectively function as two separate endocrine glands.
This article will focus on the posterior pituitary gland – its structure, function and hormones.
Structure
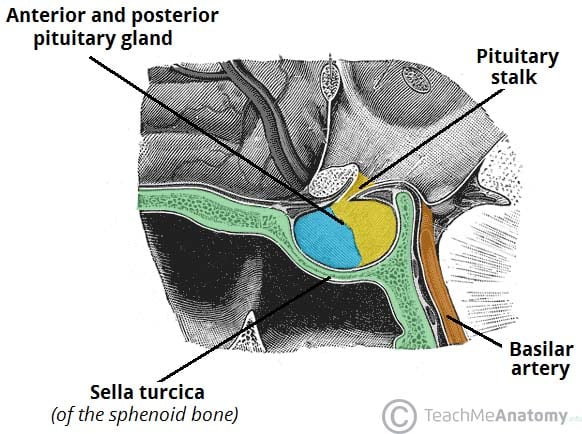
The pituitary gland (hypophysis) is a pea-sized, oval gland suspended from the brain by the pituitary stalk (infundibulum).
It is found within the sella turcica of the sphenoid bone. The posterior lobe arises from a down growth of the diencephalon – a division of the forebrain – and is essentially an extension of the hypothalamus. This lobe consists of axons of the hypothalamic neurons and neuroglial cells, which support these axons.
Hormone Release
There are two posterior pituitary hormones, oxytocin and antidiuretic hormone (ADH), which are released by periventricular and supraoptic nuclei.
These peptide hormones are derived from precursor molecules produced by hypothalamic neurons. The precursor molecules are transported along the axons of the hypothalamic neurons to the posterior pituitary. Cleavage of the precursor molecules occurs during this transport along the axons and are stored in axon terminal swellings known as Herring Bodies.
Upon appropriate stimulation, the neurosecretory neurones generate an action potential, causing the release of hormones from the nerve terminals into a rich plexus of vessels.
Oxytocin
Maternal oxytocin plasma levels gradually increase during pregnancy, and oestrogen increases the number of oxytocin receptors present in the myometrium and decidua during this time.
The primary stimulus for the release of oxytocin occurs as the cervix distends, an effect known as the Ferguson reflex. Oxytocin is an essential stimulator of myometrial contraction during labour, and uterine contraction perpetrates a positive feedback loop on oxytocin release to help maintain labour.
The release of oxytocin is also triggered by the suckling of a baby on the breast. Afferent input from the nipple triggers oxytocin production and release from the posterior pituitary into the bloodstream.
Oxytocin then travels to the myoepithelial cells of the breast to induce contraction, leading to milk expression. This is known as the milk ejection reflex or milk let-down reflex.
Antidiuretic Hormone
ADH (also called arginine vasopressin, AVP) is a neuropeptide hormone that acts on the kidney’s collecting ducts to increase water reabsorption. Various factors control ADH release, but its most important factors are changes in plasma osmotic pressure and volume status.
Osmoreceptors in the hypothalamus regulate ADH release by detecting and responding to changes in plasma osmotic pressure:
- If osmolarity increases, i.e. following a fall in plasma volume, this stimulates osmoreceptor cells to contract. This sends afferent signals from the hypothalamus to the posterior pituitary to increase the release of ADH.
- If osmolarity is decreased, i.e. following an increase in total body volume, osmoreceptors will expand. This sends afferent signals to the posterior pituitary to decrease the release of ADH.
Summary Table
Increases water permeability and absorption in collecting ducts
Clinical Relevance – Syndrome of Inappropriate Antidiuretic Hormone Secretion (SIADH)
SIADH is characterised by excessive ADH secretion.
This excessive secretion can come from the posterior pituitary or another source, such as a small cell lung carcinoma. Continual ADH production occurs independent of serum osmolality, leading to abnormally low serum sodium levels, highly osmolar urine and high urinary sodium levels. SIADH has a variety of causes, including brain injury, malignancy, drugs, infection, and hypothyroidism.
Treatment involves identifying the underlying cause and fluid restriction.
Clinical Relevance – Diabetes Insipidus
Diabetes insipidus is characterised by the passage of vast volumes of dilute urine. In some cases, as much as 20 litres of urine can be produced in 24 hours, leading to rapid dehydration and potentially death.
Neurogenic diabetes insipidus occurs due to decreased circulating levels of ADH due to impaired production/release within the central nervous system. Causes of neurogenic diabetes insipidus include:
- Mutations in vasopressin gene
- Malignancy: pituitary adenomas, craniopharyngiomas/metastases
- Trauma
- Infection: Meningitis
- Vascular: Sheehan’s Syndrome
- Sarcoidosis (formation of granulomas in the pituitary gland)
- Haemochromatosis (deposition of iron in the hypothalamus and pituitary gland)
Neurogenic diabetes insipidus can be treated with replacement therapy, such as desmopressin.
Nephrogenic diabetes insipidus occurs when ADH cannot bind to their receptors in the kidney. Causes of nephrogenic diabetes insipidus include:
- Mutations in ADH receptor gene or aquaporin-2 gene
- Metabolic: hypercalcaemia, hyperglycaemia, hypokalaemia
- Drugs
- Chronic renal disease
- Amyloidosis
Try again to score 100%. Use the information in this article to help you with the answers.
The pituitary gland is a small endocrine organ found in the brain. It consists of two lobes – the anterior and posterior pituitary. The lobes are embryologically and histologically different and effectively function as two separate endocrine glands.
This article will focus on the posterior pituitary gland – its structure, function and hormones.
Structure
The pituitary gland (hypophysis) is a pea-sized, oval gland suspended from the brain by the pituitary stalk (infundibulum).
It is found within the sella turcica of the sphenoid bone. The posterior lobe arises from a down growth of the diencephalon – a division of the forebrain – and is essentially an extension of the hypothalamus. This lobe consists of axons of the hypothalamic neurons and neuroglial cells, which support these axons.

Fig 1 – Location of pituitary gland[/caption]
Hormone Release
There are two posterior pituitary hormones, oxytocin and antidiuretic hormone (ADH), which are released by periventricular and supraoptic nuclei.
These peptide hormones are derived from precursor molecules produced by hypothalamic neurons. The precursor molecules are transported along the axons of the hypothalamic neurons to the posterior pituitary. Cleavage of the precursor molecules occurs during this transport along the axons and are stored in axon terminal swellings known as Herring Bodies.
Upon appropriate stimulation, the neurosecretory neurones generate an action potential, causing the release of hormones from the nerve terminals into a rich plexus of vessels.
Oxytocin
Maternal oxytocin plasma levels gradually increase during pregnancy, and oestrogen increases the number of oxytocin receptors present in the myometrium and decidua during this time.
The primary stimulus for the release of oxytocin occurs as the cervix distends, an effect known as the Ferguson reflex. Oxytocin is an essential stimulator of myometrial contraction during labour, and uterine contraction perpetrates a positive feedback loop on oxytocin release to help maintain labour.
The release of oxytocin is also triggered by the suckling of a baby on the breast. Afferent input from the nipple triggers oxytocin production and release from the posterior pituitary into the bloodstream.
Oxytocin then travels to the myoepithelial cells of the breast to induce contraction, leading to milk expression. This is known as the milk ejection reflex or milk let-down reflex.
Antidiuretic Hormone
ADH (also called arginine vasopressin, AVP) is a neuropeptide hormone that acts on the kidney’s collecting ducts to increase water reabsorption. Various factors control ADH release, but its most important factors are changes in plasma osmotic pressure and volume status.
Osmoreceptors in the hypothalamus regulate ADH release by detecting and responding to changes in plasma osmotic pressure:
- If osmolarity increases, i.e. following a fall in plasma volume, this stimulates osmoreceptor cells to contract. This sends afferent signals from the hypothalamus to the posterior pituitary to increase the release of ADH.
- If osmolarity is decreased, i.e. following an increase in total body volume, osmoreceptors will expand. This sends afferent signals to the posterior pituitary to decrease the release of ADH.
Summary Table
Clinical Relevance – Syndrome of Inappropriate Antidiuretic Hormone Secretion (SIADH)
SIADH is characterised by excessive ADH secretion.
This excessive secretion can come from the posterior pituitary or another source, such as a small cell lung carcinoma. Continual ADH production occurs independent of serum osmolality, leading to abnormally low serum sodium levels, highly osmolar urine and high urinary sodium levels. SIADH has a variety of causes, including brain injury, malignancy, drugs, infection, and hypothyroidism.
Treatment involves identifying the underlying cause and fluid restriction.
Clinical Relevance – Diabetes Insipidus
Diabetes insipidus is characterised by the passage of vast volumes of dilute urine. In some cases, as much as 20 litres of urine can be produced in 24 hours, leading to rapid dehydration and potentially death.
Neurogenic diabetes insipidus occurs due to decreased circulating levels of ADH due to impaired production/release within the central nervous system. Causes of neurogenic diabetes insipidus include:
- Mutations in vasopressin gene
- Malignancy: pituitary adenomas, craniopharyngiomas/metastases
- Trauma
- Infection: Meningitis
- Vascular: Sheehan’s Syndrome
- Sarcoidosis (formation of granulomas in the pituitary gland)
- Haemochromatosis (deposition of iron in the hypothalamus and pituitary gland)
Neurogenic diabetes insipidus can be treated with replacement therapy, such as desmopressin.
Nephrogenic diabetes insipidus occurs when ADH cannot bind to their receptors in the kidney. Causes of nephrogenic diabetes insipidus include:
- Mutations in ADH receptor gene or aquaporin-2 gene
- Metabolic: hypercalcaemia, hyperglycaemia, hypokalaemia
- Drugs
- Chronic renal disease
- Amyloidosis