Endocrine system 7: ovaries and testes, placenta (pregnancy)
The endocrine system comprises glands and tissues that produce hormones for regulating and coordinating vital bodily functions. This article, the seventh in an eight-part series, looks at the testes, ovaries and placenta
Abstract
The endocrine system comprises glands and tissues that produce and secrete hormones to regulate and coordinate vital functions of the human body. This article – the seventh in an eight-part series on the endocrine system – outlines the endocrine role of the ovaries and testes in producing steroid hormones that influence many areas of human physiology, as well as the endocrine role of the placenta in influencing maternal physiology and establishing the maternal-foetal interface.
Citation: Knight J et al (2021) Endocrine system 7: ovaries and testes, placenta (pregnancy). Nursing Times [online]; 117: 11, 54-58.
Authors: John Knight is associate professor in biomedical science; Zubeyde Bayram-Weston is senior lecturer in biomedical science; Maria Andrade is honorary associate professor in biomedical science; all at College of Human and Health Sciences, Swansea University.
- This article has been double-blind peer reviewed
- Scroll down to read the article or download a print-friendly PDF here (if the PDF fails to fully download please try again using a different browser)
- Click here to see other articles in this series
Introduction
The endocrine system is made up of glands and tissues that produce hormones, the role of which is to coordinate and regulate vital functions in the human body. This seventh article of an eight-part series examines the anatomy and physiology of the testes and ovaries, as well as their role in producing a variety of steroid hormones that influence many diverse areas of human physiology. We also examine the endocrine role of the placenta and how placental hormones influence maternal physiology and help establish the maternal-foetal interface.
Testes
The testes are the primary male reproductive organs, responsible for spermatogenesis (sperm production) and the synthesis and secretion of the male sex hormone, testosterone. Unlike most other major organs, they are not protected in a body cavity, but reside in a specialised pouch of skin called the scrotum. This external location is essential because optimal spermatogenesis relies on maintaining the testes’ temperature at 32-35°C; this is 2-5°C cooler than the typical core body temperature of 37°C (Durairajanayagam et al, 2014). Each testis (testicle) is oval-shaped and, in adult males, is typically 4.5-5.1cm long and weighs 15-19g (Silber, 2018). The inner structural components are protected by an outer collagen-rich capsule called the tunica albuginea (Fig 1a). Internally, each testis is divided by septa into lobules containing tightly folded seminiferous tubules; these are the site of spermatogenesis.
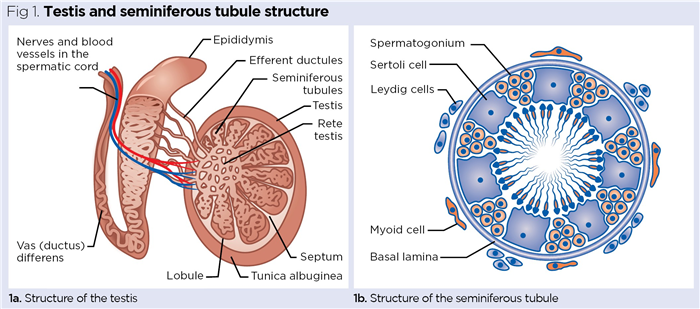
As outlined by VanPutte et al (2017) and shown in Fig 1b, three major cell types are associated with the seminiferous tubules:
Spermatogonia
Actively dividing germinal cells of the testes, spermatogonia give rise to spermatozoa that are primarily located around the inner peripheries of the seminiferous tubules. Spermatogonia initially proliferate by normal cell division (mitosis) before undergoing a special type of cell division called meiosis, which halves the number of chromosomes from the diploid number of 46 to the haploid number of 23 (Knight and Andrade, 2018). As with ova in the ovaries (discussed later in this piece), the production of haploid gametes (sex cells) is essential to restore the diploid number of chromosomes when a haploid spermatozoan fuses with a haploid ovum during fertilisation (23 + 23 = 46).
Sertoli cells
Also called nurse cells, these large cells extend from the inner periphery into the lumen of the seminiferous tubules. These cells are vital to the growth, maturation and differentiation of spermatozoa, releasing a range of nutrients and growth factors necessary for normal sperm production (França et al, 2016).
Leydig cells
Also known as interstitial cells, these are associated with the outer periphery of the seminiferous tubules. Typically spindle (diamond) shaped, they form the major endocrine cell population of the testes. They take up cholesterol and use it to synthesise testosterone, the primary male sex hormone (Zirkin and Papadopoulos, 2018).
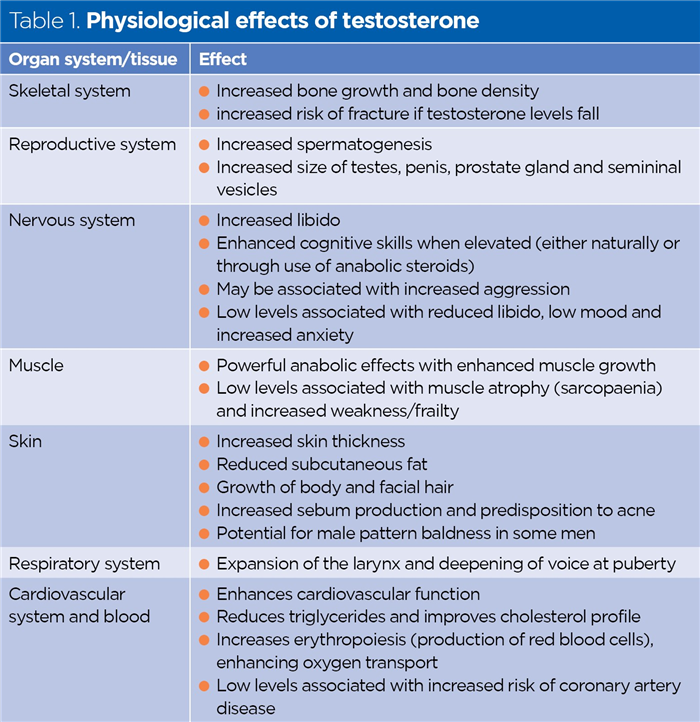
Testosterone is an anabolic steroid, largely responsible for the male secondary sexual characteristics that become apparent during puberty, including increased muscle mass, increased growth of body and facial hair, expansion of the larynx (voice breaking and deepening) and increased libido (sex drive). Testosterone has multiple effects on physiology (Table 1).
Testosterone and ageing
In most men, testosterone production begins to decline in their 30s by around 1-1.4% per year (Knight and Nigam, 2017). Known as the andropause, this often has noticably negative effects on male physiology and psychology, including increased body fat, reduced bone mass, erectile dysfunction and declining libido, poor memory and mood, reduced endurance, reduced body and facial hair, and increased lethargy (Knight and Nigam, 2017).
Ovaries
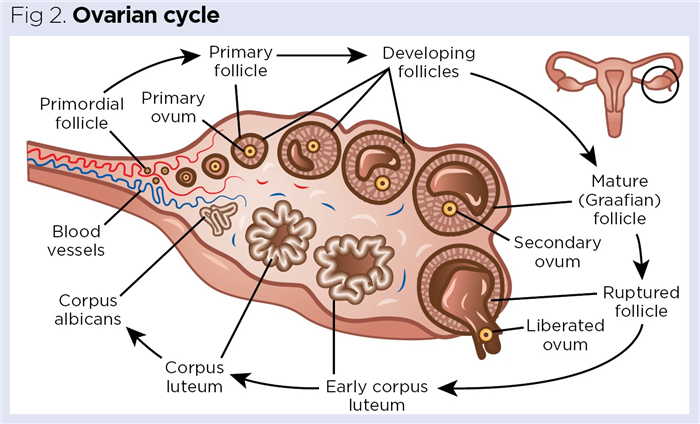
These are the primary female reproductive organs located in the pelvic cavity. They produce haploid ova (oocytes), which develop in fluid-filled sacs called follicles (Fig 2). Each mature ovary is an irregular, lumpy, almond-shaped structure, typically 3-5cm long and weighing around 5-8g (Wallace and Kelsey, 2004). The developing, and subsequently degenerating, follicles form the primary endocrine tissue in the ovaries that synthesises and secretes oestrogens and progesterone.
Menstrual cycle
The menarche (first episode of menstruation) marks the onset of puberty in females; the current average age of the menarche worldwide is around 12 years (Canelón and Boland, 2020). Most women’s menstrual cycle lasts around 28 days – the term ‘menstrual’ is derived from the Latin mensis, meaning ‘monthly’. In reality, the menstrual cycle is comprised of two cycles – the ovarian cycle and the uterine cycle – which are closely interelated.
Ovarian cycle
The ovarian cycle is the series of cyclical monthly events of follicle development and degeneration occurring in the ovaries. This consists of three distinct phases:
Follicular phase
In this phase, gonadotrophin-releasing hormone (GnRH) is released from the hypothalamus, initiating the release of follicle-stimulating hormone (FSH) from the anterior pituitary gland. FSH is a small peptide hormone that circulates in the blood and acts on the immature follicles in the ovaries. As its name implies, FSH stimulates the follicle to develop and enlarge (Orlowski and Sarao, 2021).
As the follicle grows and expands, it begins to release oestrogens (predominantly oestradiol) into the blood, with expanding follicular size correlating to increased oestrogen secretion. Gradually a fluid-filled space called the antrum develops in the follicle, increasing the pressure inside – this is known as a Graafian follicle, which is a mature follicle containing a haploid ovum (Fig 2).
Ovulation
GnRH from the hypothalamus initiates the release of luteinising hormone (LH) from the anterior pituitary gland. It is LH that initiates ovulation, with a further increase in pressure causing the ovarian follicle to rupture, propelling the ovum into the adjacent fallopian tube. Ovulation is a somewhat violent event, causing around 20% of women to experience a twinge of acute abdominopelvic pain known as mittelschmerz (German for ‘middle pain’). It is important that nurses are aware of this, as mittelschmerz can be mistaken for the symptoms of appendicitis (Durai and Ng, 2009).
Following ovulation, the remnants of the follicular walls collapse to form the corpus luteum (yellow body), which is where LH gets its name – by triggering ovulation it indirectly leads to the formation of the corpus luteum (Fig 2).
Luteal phase
Here the corpus luteum is active, with the collapsed follicular walls beginning to secrete the female sex hormone, progesterone. Progesterone’s name indicates its function in helping maintain pregnancy (pro-gestational hormone), primarily through maintaining the integrity of the endometrial (womb) lining. If fertilisation and implantation occurs, progesterone secreted by the corpus luteum helps maintain the endometrial lining for around the first 10 weeks’ gestation until the placenta takes over, secreting progesterone for the remainder of the pregnancy (Kumar and Magon 2012).
In most months, a pregnancy will not occur and the corpus luteum degenerates and shrinks into a small piece of scar tissue called the corpus albicans (Fig 2), reducing progesterone secretion and depriving the endometrium of its hormonal support. As a result, the blood vessels supplying the endometrial lining go into spasm, reducing blood flow to the endometrium. Gradually the cells of the endometrium die off, releasing internal enzymes that cause autodigestion of the functional layer, which is shed during menstruation (Bergeron, 2000).
Uterine cycle
This is the series of changes the endometrium undergoes during each 28-day cycle. Like the ovarian cycle, it has three phases:
- Menstrual phase (day 1-5) – the endometrium is deprived of progesterone, causing breakdown and shedding of the endometrial lining;
- Proliferative phase (day 6-14) – the endometrial lining is rebuilt and begins to thicken and mature. This is primarily driven by the oestrogens secreted by the developing ovarian follicles (Orlowski and Sarao, 2021);
- Secretory phase (day 14-28) – as the new endometrial lining matures, progesterone secreted by the copus luteum stimulates the endometrium to secrete a sticky mucoid material called uterine milk (Burton et al, 2007). This coats the surface of the endometrium, ensuring it is adherent, which encourages a fertilised ovum (zygote) to stick to it, helping implantation. The term ‘uterine milk’ is appropriate, as it can provide nutrition before implantation (Jones et al, 2015).
Perimenopause and menopause
Before the menopause, hormones regulating the ovarian and uterine cycles start to fluctuate, leading to progressively irregular cycles. This perimenopausal phase is highly variable in length, typically lasting between 2-10 years, and is associated with similar symptoms to the menopause itself. The average age at which women experience the menopause is 50.7 years; it affects 95% of women aged 44-56 years (Knight and Nigam, 2017). Symptoms vary significantly, but commonly include hot flushes, vaginal shrinkage and dryness, reduced bone density, sleep disturbances, mood changes and depression – mostly due to reduced levels of circulating oestrogens and progesterone. Many women are afforded relief through hormone replacement therapy, which replaces either oestrogen alone or both oestrogen and progesterone.
Hypothalamic-pituitary-gonadal axis
The release of hormones from the testes and ovaries is governed by the hypothalamic-pituitary-gonadal axis (Fig 3). The levels of testosterone and oestrogen are continually monitored by the hypothalamus and, when they begin to fall, GnRH is released from the hypothalamus into the hypothalamic pituitary portal circulation. GnRH stimulates secretion of FSH and LH from the anterior pituitary into the systemic circulation (Dwyer and Quinton, 2019).
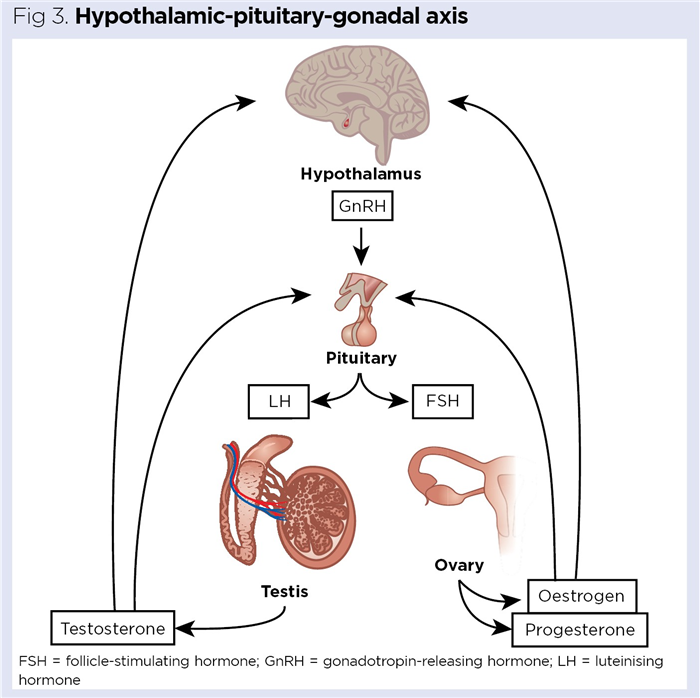
In females, FSH stimulates the enlargement of ovarian follicles, which then begin secreting oestrogen, while in males it stimulates spermatogenesis (Santi et al, 2020). LH in females stimulates ovulation; in males, it stimulates the Leydig cells to synthesise and secrete testosterone (Nedresky and Singh, 2020). As the levels of circulating oestrogen and testosterone are monitored in real time by the hypothalamus, rises in oestrogen in females and testosterone in males leads to reduced GnRH secretion from the hypothalamus; this subsequently reduces secretion of FSH and LH by the anterior pituitary.
Endocrine role of the placenta
In the first week after fertilisation, the zygote formed from the fusion of spermatozoan and ovum undergoes repeated cell divisions, resulting in a tiny hollow ball of cells called a blastocyst. The blastocyst consists of two major regions:
The placenta is a transient organ, only present for the length of the pregnancy, during which time it forms an essential bridge between the maternal and foetal circulations. The average fully formed human placenta weighs around 500g and is usually disc shaped; it is typically 22cm in diameter and 2.5cm thick (Burton and Fowden, 2015). It functions as a barrier to prevent the direct mixing of maternal and foetal blood, while simultaneously facilitating the efficient movement of:
- Oxygen and nutrients from the maternal to the foetal circulation;
- Waste products from the foetal to the maternal circulation (Burton and Fowden, 2015).
The placenta acts as a major endocrine organ throughout pregnancy, and generates several key hormones essential for a healthy gestational period and successful delivery, as described below.
Human chorionic gonadotropin
Human chorionic gonadotropin (hCG) is the hormone detected in urine and blood-based tests to confirm pregnancy. A peptide hormone, it comprises 237 amino acids and shows some structural similarities to FSH and LH. Produced and secreted by trophoblast-derived cells, hCG enhances the production of progesterone from the corpus luteum until the placenta develops sufficiently and takes over. It also stimulates angiogenesis (growth of new blood vessels) in the endometrium and is thought to modulate immune responses at the maternal/embryonic interface, allowing maternal tolerance of the developing embryo and subsequent foetus (Nwabuobi et al, 2017).
Progesterone
The production of hCG begins to wane around 6-8 weeks into pregnancy, so hCG stimulation of the corpus luteum declines. At this time, progesterone production gradually switches to the placenta, which becomes the major producer at around 10 weeks and remains so for the duration of the pregnancy (Kumar and Magon, 2012). Placental cells continually take up cholesterol from the maternal blood and this is used as the substrate to produce increasing amounts of progesterone; levels peak during the final four weeks of pregnancy, then decline after the delivery of the baby and placenta (Costa, 2016).
In addition to its key role in preventing endometrial breakdown, progesterone also:
- Inhibits uterine contraction;
- Helps ensure maternal immunotolerance of the foetus;
- Enhances proliferation of epithelial tissue in the breasts to prepare them for lactation;
- Enhances appetite during pregnancy to encourage additional calorific intake (Costa, 2016).
Leptin
Leptin is a peptide hormone consisting of 167 amino acids that has multiple physiological effects. As most leptin is secreted from fat cells (adipocytes), it is often referred to as an adopokine.
Leptin is important in regulating food intake; it is a satiety hormone that binds to receptors in the regions of the brain controlling appetite, where it reduces sensations of hunger. The placenta produces large amounts of leptin during pregnancy and it is suggested that, in some women, this may be associated with the nausea in morning sickness (Aghoozi et al, 2018).
As levels of leptin increase and peak in the second trimester of pregnancy, a tolerance to it can be estabished; this leads to increased food intake as it becomes less potent at suppressing hunger. Leptin tolerance is thought to be useful as it encourages increased calorific intake to prepare the body for the final stages of pregnancy and breast feeding (Ladyman et al, 2010).
Human placental growth hormone
Human placental growth hormone (hPGH), also known as growth hormone 2 (GH2), is structurally and functionally similar to the somatotropin (growth hormone) produced by the anterior pituitary. A peptide hormone, it consists of 191 amino acids that increase in the maternal plasma 20 weeks into gestation and peak in the final stages of pregnancy. It is thought hPGH replaces the pituitary-derived growth hormone, which typically decreases in the early-to-mid stages of pregnancy (Velegrakis et al, 2017).
The structural similarity of hPGH to pitutary-derived growth hormone (which differs by only 13 amino acids) allows it to bind to the same growth hormone receptors to illicit its biological effects (Costa, 2016); the effects of growth hormone are described in part 2 of this series.
Human placental lactogen
Like hPGH, human placental lactogen (hPL) is a peptide hormone with structural similarity to somatotropin. It is produced in increasing amounts throughout pregnancy, peaking towards the end, then declining after labour. A poorly understood hormone, it is referred to as a lactogen because it binds weakly to the same receptors as prolactin (Bayram-Weston et al, 2021) and may exert similar effects on mammary physiology. As it is structurally similar to growth hormone, it is also able to bind to growth hormone receptors and illicit growth in a similar, but weaker, way.
It has been suggested that increased production of hPL, together with other placental hormones, including progesterone and hPGH, may be associated with the insulin resistance that leads to the hyperglycaemia (increased blood sugar), observed in gestational diabetes (Plows et al, 2018).
Relaxin
Relaxin is a peptide hormone consisting of 53 amino acids produced by the corpus luteum, breast tissue and placenta. It induces a loosening of the pelvic ligaments, potentially easing delivery of the baby. This is achieved by inducing the production of enzymes, such as metalloproteases and collagenase, which function to reduce the collagen content of the ligaments, resulting in increasing laxity (Dehghan et al, 2014).
Relaxin has also been shown to have many other physiological effects. As blood volume increases by around 40-50% during pregnancy, relaxin – with progesterone and oestrogen – induces systemic vasodilation to accommodate this extra blood volume; this is usually accompanied by a characteristic drop in blood pressure in the second trimester of the pregnancy (Sanghavi and Rutherford, 2014).
Relaxin appears to work with progesterone to maintain endometrial integrity, particularly in early pregnancy, and stimulates cervical softening and ripening which are essential to the early stages of labour (Goldman and Weiss 2009).
In our final article in this series, we will examine the secondary endocrine functions of the kidneys, heart and skin.
Key points
- The testes are responsible for sperm production, and the synthesis and secretion of the male sex hormone, testosterone
- Ovarian follicles form the primary endocrine tissue of the ovaries producing the female sex hormones oestrogen and progesterone
- The menstrual cycle comprises the ovarian and uterine cycles, which are closely interrelated
- Hormone release from the testes and ovaries is ultimately controlled by the hypothalamic-pituitary-gonadal axis
- The placenta acts as a major endocrine organ throughout pregnancy, generating hormones essential for a healthy gestational period and successful delivery
Also in this series
Bayram-Weston Zet al (2021) Endocrine system 2: hypothalamus and pituitary gland. Nursing Times [online]; 117: 6, 49-53.
Bergeron C (2000) Morphological changes and protein secretion induced by progesterone in the endometrium during the luteal phase in preparation for nidation. Human Reproduction; 15: Suppl 1, 119-128.
Burton GJ, Fowden AL (2015) The placenta: a multifaceted, transient organ. Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences; 370: 1663, 20140066.
Burton GJ et al (2007) Human early placental development: potential roles of the endometrial glands. Placenta; 28: Suppl A, S64-S69.
Canelón SP, Boland MR (2020) A systematic literature review of factors affecting the timing of menarche: the potential for climate change to impact women’s health. International Journal of Environmental Research Public Health; 17: 5, 1703.
Costa MA (2016) The endocrine function of human placenta: an overview. Reproductive Biomedicine Online; 32: 1, 14-43.
Dehghan F et al (2014) The effect of relaxin on the musculoskeletal system. Scandanavian Journal of Medicine and Science in Sports; 24: 4, e220–e229.
Durai R, Ng PC (2009) Mittelschmerz mimicking appendicitis. British Journal of Hospital Medicine; 70: 7, 419.
Durairajanayagam D et al (2014) Testicular heat stress and sperm quality. In: Du Plessis SS et al (eds) Male Infertility: A Complete Guide to Lifestyle and Environmental Factors. Springer Science and Business Media.
Dwyer A, Quinton R (2019) Anatomy and physiology of the hypothalamic-pituitary-gonadal (HPG) axis. In: Llahana S et al (eds) Advanced Practice in Endocrinology Nursing. Springer.
Faghani Aghoozi M et al (2018) Investigation of the relationship between serum leptin levels and nausea and vomiting of pregnancy. Preventive Care in Nursing and Midwifery Journal; 7: 4, 64-71.
França LR et al (2016) The Sertoli cell: one hundred fifty years of beauty and plasticity. Andrology; 4: 2, 189-212.
Goldsmith LT, Weiss G (2009) Relaxin in human pregnancy. Annals of the New York Academy of Sciences; 1160: 1, 130-135.
Jones CJP et al (2015) Tracking nutrient transfer at the human maternofetal interface from 4 weeks to term. Placenta; 36: 4, 372-380.
Knight J, Andrade M (2018) Genes and chromosomes 2: cell division and genetic diversity. Nursing Times [online]; 114: 8, 40-47.
Knight J, Nigam Y (2017) Anatomy and physiology of ageing 8: the reproductive system. Nursing Times [online]; 113: 9: 44-47.
Kumar P, Magon N (2012) Hormones in pregnancy. Nigerian Medical Journal; 53: 4, 179-183.
Ladyman SR et al (2010) Hormone interactions regulating energy balance during pregnancy. Journal of Neuroendocrinology; 22: 7, 805-817.
Nedresky D, Singh G (2020) Physiology, Luteinizing Hormone. StatPearls Publishing.
Nwabuobi C et al (2017) hCG: biological functions and clinical applications. International Journal of Molecular Sciences; 18: 10, 2037.
Orlowski M, Sarao MS (2021) Physiology, Follicle Stimulating Hormone. StatPearls Publishing.
Plows JF et al (2018) The pathophysiology of gestational diabetes mellitus. International Journal of Molecular Sciences; 19: 11, 3342.
Sanghavi M, Rutherford JD (2014) Cardiovascular physiology of pregnancy. Circulation; 130: 12, 1003-1008.
Santi D et al (2020) Follicle-stimulating hormone (FSH) action on spermatogenesis: a focus on physiological and therapeutic roles. Journal of Clinical Medicine; 9: 4, 1014.
Silber S (2018) Fundamentals of Male Infertility. Springer Nature.
VanPutte CL et al (2017) Seeley’s Anatomy and Physiology. McGraw-Hill Education.
Velegrakis A et al (2017) Human placental growth hormone in normal and abnormal fetal growth (review). Biomedical Reports; 7: 2, 115-122.
Wallace WH, Kelsey TW (2004) Ovarian reserve and reproductive age may be determined from measurement of ovarian volume by transvaginal sonography. Human Reproduction; 19: 7, 1612–1617.
Zirkin BR, Papadopoulos V (2018) Leydig cells: formation, function, and regulation. Biology of Reproduction; 99: 1, 101–111.