Physiology of sweat gland function: The roles of sweating and sweat composition in human health
This is an Open Access article distributed under the terms of the Creative Commons Attribution-NonCommercial-NoDerivatives License (http://creativecommons.org/licenses/by-nc-nd/4.0/), which permits non-commercial re-use, distribution, and reproduction in any medium, provided the original work is properly cited, and is not altered, transformed, or built upon in any way.
Associated Data
ABSTRACT
The purpose of this comprehensive review is to: 1) review the physiology of sweat gland function and mechanisms determining the amount and composition of sweat excreted onto the skin surface; 2) provide an overview of the well-established thermoregulatory functions and adaptive responses of the sweat gland; and 3) discuss the state of evidence for potential non-thermoregulatory roles of sweat in the maintenance and/or perturbation of human health. The role of sweating to eliminate waste products and toxicants seems to be minor compared with other avenues of excretion via the kidneys and gastrointestinal tract; as eccrine glands do not adapt to increase excretion rates either via concentrating sweat or increasing overall sweating rate. Studies suggesting a larger role of sweat glands in clearing waste products or toxicants from the body may be an artifact of methodological issues rather than evidence for selective transport. Furthermore, unlike the renal system, it seems that sweat glands do not conserve water loss or concentrate sweat fluid through vasopressin-mediated water reabsorption. Individuals with high NaCl concentrations in sweat (e.g. cystic fibrosis) have an increased risk of NaCl imbalances during prolonged periods of heavy sweating; however, sweat-induced deficiencies appear to be of minimal risk for trace minerals and vitamins. Additional research is needed to elucidate the potential role of eccrine sweating in skin hydration and microbial defense. Finally, the utility of sweat composition as a biomarker for human physiology is currently limited; as more research is needed to determine potential relations between sweat and blood solute concentrations.
Introduction
Sweat evaporation from the skin surface plays a critical role in human thermoregulation and this is most apparent when the ability to sweat is compromised during periods of strenuous physical labor and/or exposure to hot environments [ 1 ]. For example, in anhidrotic patients [ 2 , 3 ] or individuals wearing encapsulating protective clothing/equipment [ 4 ], body core temperature rises sharply with exercise-heat stress, which can lead to heat exhaustion or heat stroke if other means of cooling are not provided. Despite the well-accepted thermoregulatory role of sweating, it is common perception that sweating has a variety of other critical homeostatic functions unrelated to thermoregulation. For instance, sweat glands are perceived to play an important excretory function, similar to that of the renal system, responsible for clearing excess micronutrients, metabolic waste, and toxicants from the body. This belief can lead individuals to engage in practices (e.g. prolonged sauna exposure, exercise in uncompensable conditions) designed to induce heavy sweat losses for their perceived health benefits. However, the effectiveness of sweat glands as an excretory organ for homeostatic purposes is currently unclear as there are no comprehensive reviews on this topic. Another common perception is that excretion of certain constituents in sweat may lead to perturbations in health, such as micronutrient imbalances. A few studies have investigated this notion but a thorough review of the literature has not been published to date. Therefore, the first aim of this paper is to provide a comprehensive review of the physiology of sweat gland function, including the types of sweat glands, their structure, and mechanisms that determine the amount and the composition of sweat excreted onto the skin surface. This will provide the background necessary to then discuss the physiological roles of sweat in the maintenance and/or perturbation of human health. In particular, this paper will provide the state of the evidence for the non-thermoregulatory as well as the thermoregulatory roles of sweating, consider the methodological challenges of studies in this area, and make suggestions where future research is needed.
Types of sweat glands
The purpose of this section is to compare and contrast the three main types of sweat glands: eccrine, apocrine, and apoeccrine [ 5 , 6 ], which are illustrated in Figure 1 . Eccrine sweat glands are the most numerous, distributed across nearly the entire body surface area, and responsible for the highest volume of sweat excretion [ 5 ]. By contrast, apocrine and apoeccrine glands play a lesser role in overall sweat production as they are limited to specific regions of the body [ 7 – 10 ]. However, it is important to briefly discuss the apocrine and apoeccrine glands since their secretions can also impact the composition of sweat collected at the skin surface.
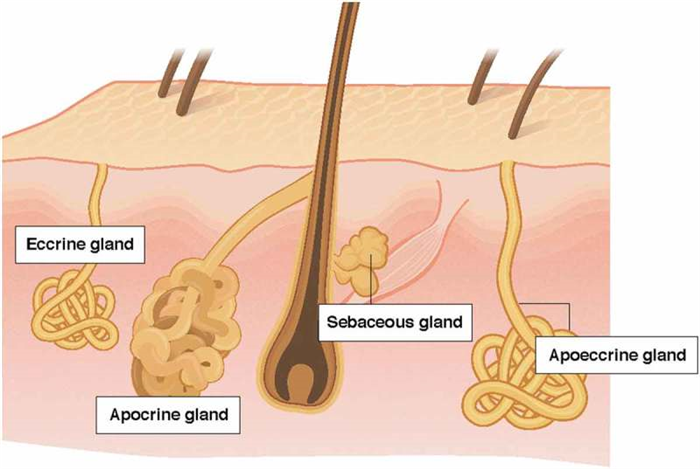
Comparison of the apocrine, eccrine, and apoeccrine glands in the axilla.
Eccrine sweat glands
Eccrine glands were the first type of sweat gland discovered; as they were initially described in 1833 by Purkinje and Wendt and in 1834 by Breschet and Roussel de Vouzzeme, but were not named eccrine glands until almost 100 years later by Schiefferdecker [ 11 ]. Eccrine glands are often referred to as the small gland variety, but are by far the most ubiquitous type of sweat gland [ 12 ]. Humans have ~2–4 million eccrine sweat glands in total and are found on both glabrous (palms, soles) and non-glabrous (hairy) skin [ 13 – 15 ]. Gland density is not uniform across the body surface area. The highest gland densities are on the palms and soles (~250–550 glands/cm 2 ) [ 16 ] and respond to emotional as well as thermal stimuli. The density of eccrine glands on non-glabrous skin, such as the face, trunk, and limbs are ~2–5-fold lower than that of glabrous skin [ 16 ], but distributed over a much larger surface area and are primarily responsible for thermoregulation.
The eccrine glands are functional early in life and, starting at ~2–3 years of age, the total number of eccrine glands is fixed throughout life [ 12 – 14 ]. Therefore, overall sweat gland density decreases with skin expansion during growth from infancy and is generally inversely proportional to body surface area. As a result, children have higher sweat gland densities than adults [ 11 ], and larger or more obese individuals have lower sweat gland densities than their smaller or leaner counterparts [ 13 , 17 ]. However, higher sweat gland density does not necessarily translate to higher sweating rate. In fact, most of the variability in regional and whole-body sweating rate within and between individuals is due to differences in sweat secretion rate per gland, rather than the total number of active sweat glands [ 18 , 19 ]. Eccrine sweat is mostly water and NaCl, but also contains a mixture of many other chemicals originating from the interstitial fluid and the eccrine gland itself. The structure and function of eccrine glands and the composition of eccrine sweat will be discussed in more detail in subsequent sections of this paper.
Apocrine sweat glands
The apocrine gland is a second type of sweat gland, which was first recognized by Krause in 1844 and later named by Schiefferdecker in 1922 [ 20 , 21 ]. Apocrine sweat glands are located primarily in the axilla, breasts, face, scalp, and the perineum [ 21 , 22 ]. As shown in Figure 1 , these glands differ from eccrine glands in that they are larger and open into hair follicles instead of onto the skin surface [ 12 ]. In addition, although present from birth, the secretory function of apocrine glands does not begin until puberty [ 23 ]. Apocrine glands produce viscous, lipid-rich sweat, which is also comprised of proteins, sugars, and ammonia [ 21 , 23 ]. The function of apocrine glands in many species is generally regarded as scent glands involved in production of pheromones (body odor), although this social/sexual function is rudimentary in humans. Apocrine gland innervation is poorly understood, but isolated sweat glands have been found to respond equally to adrenergic and cholinergic stimuli [ 23 ].
Apoeccrine sweat glands
A third type of sweat gland, only recently described by Sato et al. in 1987 [ 23 , 24 ] is the apoeccrine gland. Apoeccrine glands develop from eccrine sweat glands between the ages of ~8 to 14 years and increase to as high as 45% of the total axillary glands by age 16–18 [ 23 ]. They are intermediate in size, but as the name suggests, apoeccrine glands share properties with both eccrine and apocrine glands. Like apoeccrine glands, apoeccrine glands are limited in distribution, as they are contained to only the axillary region. Apoeccrine glands are more similar to eccrine glands in that the distal duct connects to and empties sweat directly onto skin surface [ 23 ]. In addition, the apoeccrine gland produces copious salt water secretions similar to eccrine sweat [ 23 ]. The function of this secretion is unknown, but unlikely to play a significant role in thermoregulation since evaporation is inefficient in the axilla region. The innervation of the apocrine gland is still poorly understood, but in vitro models suggest the apocrine gland is more sensitive to cholinergic than adrenergic stimuli [ 23 , 24 ].
Sebaceous glands
Sebaceous glands are not a type of sweat gland but worth mentioning here since their secretions can impact the composition of sweat collected at the skin surface [ 25 ]. Sebaceous glands, first described by Eichorn in 1826 [ 26 ], are associated with hair follicles and present over much of the body surface but particularly the scalp, forehead, face, and anogenital area [ 26 , 27 ]. They are absent on the palms of hands and soles of the feet [ 26 ]. Sebaceous glands are holocrine glands that secrete a viscous, lipid-rich fluid consisting of triglycerides, wax esters, squalene, cholesterol, and cholesterol esters [ 25 – 27 ]. The rate of sebum production is related to the number and size of glands which is under hormonal (androgen) control [ 26 ]. The importance of sebaceous gland secretions is uncertain but sebum is thought to have antibacterial and antifungal properties and function as a pheromone [ 28 ].
Eccrine glands will be the focus of this review; therefore, unless otherwise specified, sweating rate and sweat composition will hereafter refer to that of the eccrine glands. The reader is referred to other papers for more details on apocrine and apoeccrine glands [ 12 , 20 – 24 , 27 , 29 , 30 ] as well as sebaceous glands [ 26 – 28 ].
Structure and function of eccrine sweat glands
Anatomy
The anatomical structure of the eccrine sweat gland, illustrated in Figure 2 , consists of a secretory coil and duct made up of a simple tubular epithelium. The secretory tubule is continuous with and tightly coiled with the proximal duct. The distal segment of the duct is relatively straight and connects with the acrosyringium in the epidermis [ 5 ]. The secretory coil has three types of cells: clear, dark, and myoepithelial. As shown in Figure 2(c ), clear cells are responsible for the secretion of primary sweat, which is nearly isotonic with blood plasma [ 6 – 8 ]. The clear cells contain a system of intercellular canaliculi, glycogen, and a large amount of mitochondria and Na-K-ATPase activity [ 5 ]. The dark cells are distinguishable by the abundance of dark cell granules in the cytoplasm. Their function is poorly understood, but thought to potentially act as a repository for various bioactive materials involved in regulation of clear cell and duct cell function [ 9 , 10 ]. The function of the myoepithelial cells is provision of structural support for the gland against the hydrostatic pressure generated during sweat production [ 5 ]. The duct has two cell layers: basal and luminal cells. Its primary function is reabsorption of Na and Cl ions as sweat flows through the duct, as shown in Figure 2(d ). Most of the NaCl reabsorption occurs in the proximal duct, as these cells contain more mitochondria and Na-K-ATPase activity than that of the distal segment of the eccrine duct [ 5 ]. The result is a hypotonic final sweat excreted onto the skin surface [ 6 , 9 ].
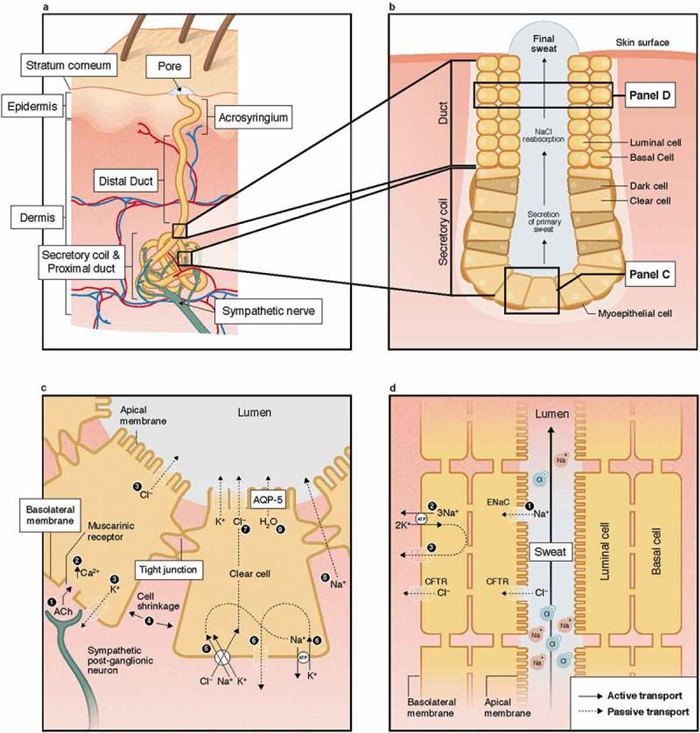
Structure of the eccrine sweat gland (panels A-B) and mechanisms of sweat secretion in the secretory coil (panel C) and Na and Cl reabsorption in the proximal duct (panel D). ACh; acetylcholine; AQP-5, aquaporin-5; CFTR, cystic fibrosis membrane channel; ENaC, epithelial Na channel; NaCl, sodium chloride.
Mechanisms of secretion and reabsorption
Secretion
The basic mechanism by which secretion of primary sweat occurs in the clear cells, according to the Na-K-2Cl cotransport model, is illustrated in Figure 2(c ). First, binding of acetylcholine to muscarinic receptors on the basolateral membrane of the clear cell triggers a release of intracellular Ca stores and an influx of extracellular Ca into cytoplasm. This is followed by an efflux of KCl through Cl channels in the apical membrane and K channels in the basolateral membrane. This leads to cell shrinkage, which triggers an influx of Na, K, and Cl via Na-K-2Cl cotransporters on the basolateral membrane and subsequently Na and K efflux via Na-K-ATPase and K channels on basolateral membrane as well as Cl efflux via Cl channels on apical membrane. Increased Cl concentration in the lumen creates an electrochemical gradient for Na movement across the cell junction [ 9 , 10 ]. In turn, the net KCl efflux from the cell creates an osmotic gradient for water movement into the lumen via aquaporin-5 channels [ 31 – 33 ].
Ion reabsorption
Figure 2(d ) shows the mechanism of ion reabsorption according to the modified Ussing leak-pump model. On the apical membrane of the luminal cells passive influx of Na occurs through amiloride-sensitive epithelial Na channels. Active transport of Na across the basolateral membrane of the basal cells occurs via Na-K-ATPase, which is accompanied by passive efflux of K through K channels on the basolateral membrane. The movement of Cl is largely passive via cystic fibrosis membrane channels (CFTR) on both the apical and basolateral membranes [ 9 , 34 , 35 ]. The two cell layers are thought to be coupled and behave like a syncytium. The sweat duct also reabsorbs bicarbonate, either directly or through hydrogen ion secretion, but the specific mechanism is unknown [ 5 , 8 , 36 , 37 ]. The activity of Na-K-ATPase is influenced by the hormonal control of aldosterone [ 38 ]. Overall the rate of Na, Cl, and bicarbonate reabsorption is also flow-dependent, such that higher sweating rates are associated with proportionally lower reabsorption rates resulting in higher final sweat electrolyte concentrations [ 39 , 40 ]. This concept will be covered in more detail in the Effect of sweat flow rate section below.
Sweat gland metabolism
Transport of Na across cellular membranes is an active process, thus sweat secretion in the clear cells and Na reabsorption in the duct require ATP. The main route of energy production for sweat gland activity is oxidative phosphorylation of plasma glucose [ 6 , 41 ]. Cellular glycogen is also mobilized in the eccrine sweat gland during sweat secretion, but its absolute amount is too limited to sustain sweat secretion. Thus, the sweat gland depends almost exclusively on exogenous substrates, especially glucose, as its fuel sources [ 6 , 42 ]. Although the sweat gland is capable of utilizing lactate and pyruvate as energy sources, these intermediates are less efficient than glucose [ 6 , 9 ]. Indeed, studies have shown that arterial occlusion of forearms [ 43 , 44 ] and removal of glucose and oxygen from the bathing medium of isolated sweat glands [ 6 , 45 ] inhibits sweat production. Consequently, lactate (as an end product of glycolysis) and NaCl concentrations in sweat rise sharply. Taken together, these results indicate that oxygen supply to the sweat gland is important for maintaining sweat secretion and ion reabsorption [ 45 ].
Control of eccrine sweating
Eccrine sweat glands primarily respond to thermal stimuli; particularly increased body core temperature [ 40 ], but skin temperature and associated increases in skin blood flow also play a role [ 9 , 46 – 49 ]. An increase in body temperature is sensed by central and skin thermoreceptors and this information is processed by the preoptic area of the hypothalamus to trigger the sudomotor response. Recent studies suggest that thermoreceptors in the abdominal region [ 50 , 51 ] and muscles [ 52 ] also play a role in the control of sweating. Thermal sweating is mediated predominately by sympathetic cholinergic stimulation. Sweat production is stimulated through the release of acetylcholine from nonmyelinated class C sympathetic postganglionic fibers, which binds to muscarinic (subtype 3) receptors on the sweat gland (see Figure 2(c )) [ 9 ]. Eccrine glands also secrete sweat in response to adrenergic stimulation, but to a much lesser extent than that of cholinergic stimulation [ 6 , 53 ]. Catecholamines, as well as other neuromodulators, such as vasoactive intestinal peptide, calcitonin gene-related peptide, and nitric oxide, have also been found to play minor roles in the neural stimulation of eccrine sweating [ 9 , 54 , 55 ]. In addition, eccrine sweat glands respond to non-thermal stimuli related to exercise and are thought to be mediated by feed-forward mechanisms related to central command, the exercise pressor reflex (muscle metabo- and mechanoreceptors), osmoreceptors, and possibly baroreceptors [ 55 , 56 ].
Sweating rate over the whole body is a product of the density of active sweat glands and the secretion rate per gland. Upon stimulation of sweating, the initial response is a rapid increase in sweat gland recruitment, followed by a more gradual increase in sweat secretion per gland [ 13 , 57 – 59 ]. Two important aspects of thermoregulatory sweating, depicted in Figure 3 , are the onset (i.e. body core temperature threshold) and sensitivity (i.e. slope of the relation between sweating rate and the change in body core temperature) of the sweating response to hyperthermia [ 60 ]. Shifts in the sweating temperature threshold are thought to be central (hypothalamic) in origin, whereas changes in sensitivity are peripheral (at the level of sweat glands) [ 61 ].
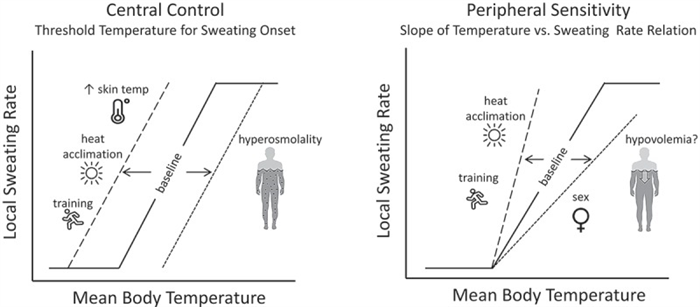
An illustration of central and peripheral control of sweating and the factors that modify the sweating response to hyperthermia. Shifts in the onset (threshold) and sensitivity (slope) of the sweating response to hyperthermia are depicted by the dashed lines. Other potential factors that may directly or indirectly modify sweating (altitude/hypoxia, microgravity, menstrual cycle, maturation, aging) are discussed in the text.
Modifiers of eccrine sweating
Several intra- and interindividual factors can modify the control of sweating [ 60 ], some of which are shown in Figure 3 . For example, the enhancement of sweating with heat acclimation [ 62 – 65 ] and aerobic training [ 66 – 69 ] has been associated with both an earlier onset and greater responsiveness of sweating in relation to body core temperature [ 64 , 70 – 75 ]. By contrast, dehydration has been shown to delay the sweating response [ 76 , 77 ], as hyperosmolality increases the body temperature threshold for sweating onset [ 78 – 81 ]. Hypovolemia may reduce sweating sensitivity [ 82 ], but this finding has not been consistent [ 79 , 83 ].
Other examples of host and external factors that modify regional and/or whole-body sweating are provided in Table 1 . For example, older adults exhibit a lower sweat output per activated gland in response to a given pharmacological stimulus or passive heating compared with younger adults [ 84 – 86 ]. This decline in sweating occurs gradually throughout adulthood [ 85 , 87 ] and there are regional differences in the age-related decrement in sweat gland function [ 88 – 91 ]. However, the decline in sweating rate with aging has been primarily attributed to mechanisms related to a decline in aerobic fitness and heat acclimation (possibly due to decreased sensitivity of sweat glands to cholinergic stimulation [ 67 , 92 ]), rather than age per se [ 67 , 68 , 85 , 93 – 96 ]. In addition, lifetime ultraviolet exposure and other environmental factors may have an interactive effect with chronological age in determining sweat gland responsiveness [ 84 ]. Nonetheless, it is important to note that most studies have reported no significant difference in sweating between older and younger adults during exercise in the heat; with the exception of peak sweating rates associated with hot dry climates [ 94 , 97 , 98 ]. Therefore the ability of older adults to maintain body core temperature during heat stress is usually not compromised. When the effects of concurrent factors, such as fitness level, body composition, and chronic disease are removed, thermal tolerance appears to be minimally compromised by age [ 93 ].
Table 1.
Host and environmental factors that modify sweat gland function.
| Timing | Effect on sweating rate and/or sweat composition | |
|---|---|---|
| Host and external factors | ||
| Dietary NaCl | Acute/ Chronic |
No effect on sweating rate [ 224 ,236,242–244]; mixed results for effect on sweat [Na] and [Cl] (see Table 5 ): most studies involving several days to weeks of dietary Na manipulation are associated with significant changes in sweat [Na] [ 224 ,235,236,239,240], but there is no correlation between the change in Na intake and the change in sweat [Na] [ 247 ]; shorter duration (≤3 days) of Na manipulation has minimal or no impact [ 134 ,195,242,244,245] |
| Dietary intake of other minerals (Ca, Fe, Zn, Cu) and vitamins (ascorbic acid, thiamine) | Acute/ Chronic |
No effect on sweat mineral or vitamin concentrations [ 230 ,231,248–250] |
| Fluid intake | Acute | Water ingestion results in a reflex (oropharyngeal) transient increase in RSR, especially when in a hypohydrated state [ 360 ,361]; no effect on sweat Na, K, Cl, and lactate concentrations [ 361 ] |
| Dehydration | Acute | Reduced WBSR and RSR attributed to hyperosmolality-induced increase in threshold for sweat onset and to a lesser extent by a hypovolemia-induced decrease in sweat sensitivity (see Figure 3 ) [ 42 ,76–78,80,82]; equivocal effects on sweat [Na] and [Cl] [ 134 ,153,190–195]; no effect on sweat [K] [ 190 ] |
| Alcohol | Acute | No effect on sweating rate [ 331 ,332]; sweat ethanol concentration increases with ethanol ingestion and rises linearly with increases in blood alcohol concentration [ 334 ,335] |
| Exercise Intensity | Acute | Increase in WBSR and RSR with increases in exercise intensity [ 104 ,362] as metabolic heat production is directly proportional to energy expenditure [ 201 ,203]; sweat [Na] and [Cl] increase with increases in exercise intensity because the relative rate of Na and Cl reabsorption is flow dependent [ 39 ,159], minimal or no effect on sweat [K] [ 159 ], inverse relation between sweating rate and sweat lactate [ 162 ] and ammonia concentrations [ 6 ,15] (see Table 4 ), limited data on other sweat constituents |
| Environment | Acute | Increase in WBSR and RSR [ 201 ,363–368] with increased environmental heat stress (increased air temperature, increased solar radiation, decreased air velocity) at a given workload; suppression of sweating via hidromeiosis with prolonged exposure to humid/still air [ 366 ,369–371]. Increase in sweat [Na] and [Cl] with an increase in ambient temperature [ 158 ,372] |
| Altitude/hypoxia | Acute | Equivocal effects on sweating; reduced sweat sensitivity for RSR [ 118 ,119,373], but mixed results for WBSR [ 374 –378]. Limited data on sweat composition. |
| Clothing/protective equipment | Acute | Increase in WBSR and RSR because of reduced evaporative and radiant heat loss from covering the skin surface, heavy protective gear can also increase metabolic heat production [ 4 ,379,380]; limited data on sweat composition |
| Body mass | Chronic | Increased WBSR in individuals with larger body mass because of increased metabolic heat production at a given absolute workload during weight-bearing exercise [ 381 –383] and possibly lower sweating efficiency [ 384 ]; limited data on sweat composition |
| Heat acclimation | Chronic | Increase in WBSR [ 64 ,220,222,385], variable effects at the regional level such that RSR at the limbs (forearm) tend to increase proportionally more than at central sites (chest, back), suggesting a possible preferential redistribution toward the periphery resulting in more uniform sweating across the body [ 221 ,222]; reduced sweat [Na] and [Cl] [ 62 ,63,158,217,218,220], but no change in other minerals (K, Mg, Ca, Fe, Cu, Zn) [ 227 ]. Sudomotor changes due to gland hypertrophy, increased cholinergic and aldosterone sensitivity, and decreased threshold for sweat onset (see Figure 3 ). |
| Aerobic training | Chronic | Increase in WBSR and RSR because of increased cholinergic sensitivity and decreased threshold for sweat onset [ 66 –69] (see Figure 3 ); limited data for sweat composition |
| Sex | Chronic | Higher WBSR and RSR in men because of greater cholinergic responsiveness (see Figure 3 ) and maximal sweating rate, but only at high evaporative requirements for heat balance [ 83 ,99–102]; otherwise higher WBSR often observed in men are related to higher body mass and metabolic heat production, rather than sex per se [ 104 –108]. Minimal differences in sweat [Na], [Cl], and [lactate] due to sex per se [ 103 ,134,156,386–388]; limited data on other constituents |
| Menstrual cycle | Cyclical | No effect on WBSR [ 123 ,126–128], but lower RSR at a given body core temperature (increased threshold and decreased slope) during luteal phase [ 122 –125]; no effect on sweat [Na], [Cl], or [K] [ 389 ] |
| Circadian Rhythm | Cyclical | Increased sweating threshold in the afternoon (1200–1600 h) vs. early morning (400–530 h) [ 121 ,122]; no data on sweat composition |
| Race/Ethnicity | Chronic | No inherent race or ethnicity differences in WBSR, RSR, or sweat composition [ 134 ,390,391]; but heat habituation, characterized by lower (lower ASGD and SGO) and more efficient sweating (less dripping), occurs in people indigenous to hot or tropical climates [ 70 ,392–397] |
| Maturation | Progressive change | Lower WBSR and sweat [Na] in pre-pubertal vs. post-pubertal boys [ 94 ,115–117] |
| Aging | Progressive change | Reduced WBSR and RSR related to decreased SGO associated with decline in aerobic fitness and heat acclimation rat her than aging per se [ 67 ,85,93,94]; during exercise-heat stress differences more evident for peak sweating rate (e.g. during exercise in hot dry climates) [ 97 ]. Limited data on sweat composition, but there seems to be no impact of age per se on sweat [Na] [ 67 ]. |
ASGD: activated sweat gland density; RSR: regional sweating rate; SGO: sweat gland output; WBSR:, whole-body sweating rate.
Table 2.
Overestimation (by up to 2–3x) of micronutrient concentrations, but negligible effect on Na and Cl [ 134 , 183 , 227 , 228 ]
Overestimation of lipophilic compounds abundant in secretions from sebaceous glands (e.g. cytokines, lactate, vitamins, persistent organic pollutants) [ 25 , 316 , 318 ]
Avoid sebum contamination by collecting sweat from sites with fewer sebaceous glands and using absorbent pad technique [ 398 ]
Sweat collected at the onset of exercise includes skin surface contamination from residual sweat in ductal lumen [ 234 , 399 ]
Sweat collection at onset of exercise (when sweating rate is low) not representative of sweat electrolyte concentrations at steady state sweating rate.
Overestimation (by 1.2-5x) of trace minerals (Fe, Ca, Zn, Mg, Cu) and most other constituents (urea, ammonia, lactate, cytokines, amino acids) compared with later in exercise [ 228 , 229 , 232 – 234 , 239 , 399 , 400 ], but negligible effect on Na, Cl, and K [ 227 – 229 ]
Use regional method such as absorbent patch for ease of application with athletes and to avoid contamination [ 156 ]
Collect sweat during exercise representative of training/competition intensities and environmental conditions [ 132 , 159 ]
Creates microenvironment (increases local skin temperature and humidity) [ 362 ] and can alter regional sweating rate compared with uncovered skin [ 370 , 410 ]
Contaminants from the environment cannot penetrate adhesive barrier (Tegaderm TM ) so can be worn during normal activities, including exercise and swimming [ 412 ].
Takes long time (>60 min) to collect enough sweat for analysis (0.5 mL capacity for Megaduct) [ 414 ]
Forced ventilation and maintenance of dry skin facilitates higher RSR under the capsule than surrounding skin (at least in a humid/still ambient air) [ 415 , 416 ]
Particularly susceptible to skin surface contamination due to desquamation and difficulty in cleaning irregular surfaces of hand [ 228 ]
Artificial elevation of concentrations of contaminants of epidermal origin (e.g. by 20x for aminopeptidase) [ 423 ]
Criterion laboratory-based methods are ion chromatography, inductively coupled mass spectroscopy, flame atomic emission, or absorption spectrometry [ 430 – 432 ]
Table 3.
Sweat micronutrients: Mechanisms and methodological considerations.
| Concentration in sweat | Comparison of regional and whole body sweat | Correlation between sweat and blood | Sweat gland mechanisms | Potential methodological Issues | |
|---|---|---|---|---|---|
| Sodium | 10–90 mmol/L [ 145 –148,156] | Significant correlation; many (forehead, back, chest, upper arm) but not all (foot, calf, thigh) regional sites overestimate whole body concentrations [ 146 ,147,149,159] | a [ 228 , 295 ]; plasma [Na] influences sweat [Na], but not reliably correlated because of other factors that dictate reabsorption rates | Secreted via paracellular transport [ 15 ]. Primary sweat is nearly isotonic with blood plasma [ 8 ,307,434]; Na is reabsorbed in the duct via epithelial Na channels and Na-K-ATPase (activity influenced by aldosterone) [ 38 ] transporters [ 9 ,34] (see Figure 2 ), resulting in hypotonic final sweat | Concentration varies (up to 2–3 fold) with sweating rate [ 6 ,39,40,159] |
| Chloride | 10–90 mmol/L [ 147 –149,152,159,435] | Significant correlation; many (forehead, back, chest, upper arm) but not all (foot, calf, thigh) regional sites overestimate whole body concentrations [ 146 ,147,149,159] | a [ 152 , 195 ]; plasma [Cl] influences sweat [Cl], but not reliably correlated because of other factors that dictate reabsorption rates | Secreted via Na-K-2Cl cotransport model [ 15 ]. Primary sweat is slightly hypertonic compared with blood plasma [ 8 ,434]; Cl is reabsorbed in the duct via the CFTR [ 35 ] (see Figure 2 ), resulting in hypotonic final sweat | Concentration varies (up to 2-3 fold) with sweating rate [ 6 ,152,159] |
| Potassium | 2–8 mmol/L [ 146 ,147,435] | Mixed results with respect to correlation [ 146 ,147,149,159] | a [ 228 ] | Secreted via Na-K-2Cl cotransport model [ 15 ]. Primary sweat is nearly isotonic with blood plasma [ 6 ,8,434]; Mixed results with respect to relation between flow rate and sweat [K] [ 6 ,147,159,436]; thought to be secreted during sweat passage along the duct, but mechanism unknown [ 437 –439] | Often overestimated (by up to 2-3x) with arm bag technique due to surface contamination [ 134 ,228] |
| Calcium | b 0.2–2.0 mmol/L [ 144 ,219,227–229,235,286] | No correlation; regional measures overestimate whole body concentrations [ 286 ] | No [ 283 ] | NA | Overestimation (by up to 3x) both of epidermal origin and residual Ca in the sweat gland lumen [ 134 ,228] |
| Magnesium | b 0.02–0.40 mmol/L [ 219 ,227–229] | No correlation; regional measures overestimate whole body concentrations [ 286 ] | NA | NA | Overestimation (by up to 3x) from skin surface contamination [ 228 ] |
| Iron | b 0.0001–0.03 mmol/L [ 219 ,228,233,234,274] | Regional measures overestimate whole body concentrations [ 285 ] | No [ 233 ,251] | NA | Overestimation (by up to 2-3x) both of epidermal origin and residual Fe in the sweat gland lumen [ 134 ,228,233,234]; cell-rich sweat particularly high in Fe [ 184 ,233,234,253,254] |
| Zinc | b 0.0001–0.02 mmol/L [ 219 ,227–229,274] | Regional measures overestimate whole body concentrations [ 285 ] | NA | NA | Overestimation (by up to 2x) from skin surface contamination [ 228 ] |
| Copper | b 0.0005–0.02 mmol/L [ 219 ,227–229,274,285,286] | No correlation; regional measures overestimate whole body concentrations [ 285 ,286] | NA | NA | Overestimation (by up to 3.5x) from skin surface contamination [ 228 ] |
| Vitamins | a | NA | NA | NA | Overestimation from skin surface contamination and sebum secretions [ 25 ,134] |
NA: no data available; a mixed results or too few studies available to draw conclusion; b values are from regional or whole-body sweat reported from studies that took measures to prevent epidermal contamination (e.g. pre-rinsed skin and analyzed cell-free sweat)
Table 4.
Selected non-micronutrient components of eccrine sweat: Mechanisms and methodological considerations.
| Concentration in sweat | Comparison of regional and whole body sweat | Correlation between sweat and blood | Sweat gland mechanisms | Functional role in eccrine sweat | Potential methodological Issues | |
|---|---|---|---|---|---|---|
| Lactate | 5–40 mmol/L [ 6 ,45,147,162,440–443] | No correlation [ 147 ] | No [ 45 ,181,440–442] | Produced by eccrine sweat gland metabolism [ 13 ,15,45,134,140]. Inverse relation between sweating rate and sweat lactate concentration (dilution effect), but direct relation between sweating rate and lactate excretion rate [ 161 –163]. | Natural skin moisturizer [ 15 ,214] Excretion of metabolic waste – not enough evidence [ 440 ] |
Concentration varies with changes in sweating rate. Skin surface contamination from residual lactate in sweat ducts [ 163 ,442] |
| Urea | 4–12 mmol/L [ 45 ,193,441] | Significant correlation, but regional measures overestimate whole body concentrations [ 444 ] | a [ 337 , 339 , 441 ] | Primarily derived from plasma [ 239 ]. Readily crosses glandular wall and cell membrane and therefore concentrations expected to be same as or slightly higher than plasma [ 15 ]. However, measured concentrations are often significantly higher in sweat than plasma [ 337 –339]; possibly because synthesis of urea by the gland [ 6 ] or surface contamination issues. | Natural skin moisturizer [ 15 ] Excretion of metabolic waste – not enough evidence [ 134 ] |
Concentration changes with variation in sweating rate [ 6 ]. Skin surface contamination from residual urea in sweat gland lumen [ 183 ,239] |
| Ethanol | 2–30 mmol/L [ 334 ,335] | NA | Yes [ 334 ,335] | Primarily derived from plasma [ 334 ,335]. | Detoxification – not enough evidence [ 336 ] | Evaporation of ethanol during sweat collection [ 401 ] |
| Ammonia | 1–8 mmol/L [ 15 ,193,400,441] | Regional measures overestimate whole body concentrations [ 445 ] | a [ 400 , 441 ] | Concentrations 20-50x that of plasma and is inversely related to sweating rate and pH. Primarily derived from plasma NH3 by nonionic passive diffusion of NH3 to acidic ductal sweat and ionic trapping of NH4 [ 6 ,15]. | Excretion of metabolic waste – not enough evidence [ 134 ,193] | Skin surface contamination from residual NH3 in sweat gland lumen and/or breakdown of urea by bacteria on skin [ 400 ] |
| Bicarbonate | 0.5–5.0 mmol/L [ 147 ,443] | NA | a [ 443 ] | Primary fluid in secretory coil is lower than blood plasma [ 5 , 434 ]. HCO3 is reabsorbed in the sweat duct (via CFTR directly [ 36 ] or via H + secretion), resulting in acidification of final sweat [ 36 ]. HCO3 reabsorption is inversely related to sweating rate (i.e. reabsorption is higher at low sweating rate). Thus final sweat pH is lower (more acidic) at lower sweating rate [ 5 , 8 , 37 , 160 ] | Dictates pH of sweat [ 5 ,8] | Concentration varies with changes in sweating rate [ 37 ,160] |
| Glucose | 0.01–0.20 mmol/L [ 183 ,446,447] | NA | a [ 134 , 446 – 448 ] | Secreted via paracellular transport [ 449 ]. Plasma glucose is the primary energy source for eccrine sweat gland secretory activity [ 6 ,41]. | NA specific to its presence in sweat | Possible skin surface contamination from residual glucose in sweat ducts |
| Heavy Metals (e.g. arsenic, lead, mercury, cadmium) |
Lead 0.00002–0.00006 mmol/L [ 285 ,325] | Regional measures overestimate whole body concentrations [ 285 ] | No [ 318 ,325] | Concentrations are often significantly higher in sweat than plasma [ 280 ,314,318], but no known mechanisms for preferential secretion. | Detoxification – not enough evidence [ 325 ] | Skin surface contamination from epidermis and/or sebum secretions [ 318 ,321,322] |
| Antibodies (e.g. IgG, IgA) and Antimicrobial peptides (e.g. dermcidin, cathelicidin, lactoferrin) | a | NA | NA | Mechanism of secretion unclear [ 450 ]; produced by eccrine sweat gland [ 211 –214] | Protect against infections by controlling certain pathogenic bacterial counts on skin surface [ 211 –214] | Skin surface contamination from residual antibodies and antimicrobial peptides in sweat ducts |
| Other Proteins (e.g. albumin, α-globulin, γ-globulin) | a | NA | NA | Paracellular transport, but pathway not fully understood; thought to involve tight-junction remodeling [ 451 ] | NA specific to its presence in sweat | Concentration varies with sweating rate [ 15 ]. Potential for contamination by epidermal protein [ 6 ]. |
| Cytokines (e.g. Interleukin-1α, 1β, 6, 8, 31, TNFα) |
a | NA | a [ 452 , 453 ] | Derived from eccrine sweat gland (stress-induced increased secretion of Interluekin-1) and plasma [ 10 ,399,454,455]. Concentrations increase with increasing sweating rate [ 399 ]. | NA specific to its presence in sweat | Skin surface contamination, both of epidermal origin and residual cytokines in the sweat gland lumen [ 454 ] |
| Amino acids (e.g. pyrrolidone carboxylic acid, urocanic acid, serine, histadine, ornithine, glycine, alanine, aspartic acid, lysine) |
a | NA | NA | Secretory mechanisms are unknown [ 6 ]. Present in sweat but varied in concentrations perhaps because of contamination [ 15 ] | Natural skin moisturizers, maintain barrier integrity of skin [ 206 ,207] | Skin surface contamination, both of epidermal origin and residual amino acids in the sweat gland lumen [ 15 ,136,207] |
| Proteolytic enzymes | a | NA | NA | Derived from eccrine sweat gland [ 10 ,456] and/or epidermis [ 423 ]. Concentrations increase with increasing sweating rate [ 457 ]. | Skin maintenance and protection via desquamation of horny layer, hydrolysis of debris in the ductal lumen, allergen inhibition [ 456 ] | Skin surface contamination, both of epidermal origin [ 423 ] and residual proteolytic enzymes in the sweat gland lumen [ 457 ] |
| Persistent Organic Pollutants (e.g. organochlorinated pesticides, polychlorinated biphenyls, perfluorinated compounds) |
a | NA | No [ 312 ,316,458] | Concentrations are often significantly higher in sweat than plasma [ 312 ,316], but no known mechanisms for preferential secretion. Persistent organic pollutants are lipophilic and thus may appear on skin surface through sebum secretions [ 458 ]. | Detoxification – not enough evidence [ 458 ] | Skin surface contamination from epidermis and/or sebum secretions [ 323 ,458] |
| Other toxicants (e.g. BPA, phthalate, polybrominated diphenyl ethers) |
a | NA | No [ 311 ,313,317] | Concentrations are often significantly higher in sweat than plasma [ 311 ,313,317], but no known mechanisms for preferential secretion. | Detoxification – not enough evidence [ 323 ,324] | Skin surface contamination from epidermis and/or sebum secretions [ 323 ] |
BPA: bisphenol-A; NA: no data available; HCO3: bicarbonate; NH3: ammonia; a mixed results or too few studies available to draw conclusion.
Table 5.
Studies on sodium intake and sweat electrolyte concentration and total electrolyte loss during exercise and/or heat stress.
Increase during DEH/NaCl maintained trial (25 to 32 mM Cl); increase started at 14 h and continued thru 25 h
Decrease during EUH/NaCl depletion trial (28 to 17 mM Cl); decline started at 14 h and continued thru 25 h.
It is often reported that men exhibit higher sweating rates than women; and several factors, some of which are independent effects of sex and others due to confounding physical characteristics, seem to contribute depending upon the study design. Men have a greater cholinergic responsiveness (see Figure 3 ) and maximal sweating rate than women [ 83 , 99 – 101 ]. However, studies in which subjects were matched for body mass, surface area, and metabolic heat production, have shown that sex differences in whole-body sweat production are only evident above a certain combination of environmental conditions (e.g. 35–40°C, 12% rh) and rate of metabolic heat production (e.g. 300–500 W/m 2 ) leading to high evaporative requirements for heat balance [ 83 , 100 – 102 ]. Sweat gland density is generally higher in women than men (due in part to lower body surface area) [ 17 , 69 , 103 ]. Accordingly, the lower sweating rates by women reported in some studies were a result of lower output per gland [ 99 , 101 , 103 ]. Otherwise, higher whole-body sweating rates observed in men than women (e.g. in cross-sectional studies) can usually be attributed to higher body mass and metabolic heat production (higher absolute exercise intensities), rather than sex per se [ 104 – 109 ]. Taken together it seems that women are not at a thermoregulatory disadvantage compared with men for most activities and environmental conditions typically encountered [ 110 , 111 ]. As discussed in more detail elsewhere [ 109 , 110 , 112 ], other factors such as body size, surface area-to-mass ratio, heat acclimation status, aerobic capacity, exercise intensity, and environmental conditions (all of which directly or indirectly impact the evaporative requirement for heat balance) are more important than sex in determining sudomotor responses to exercise-heat stress. The reader is referred to published reviews for more comprehensive discussions on the effects of sex and sex hormones on thermoregulation [ 110 , 113 , 114 ].
Additional factors, such as maturation [ 94 , 115 – 117 ], altitude/hypoxia [ 118 – 120 ], circadian rhythm [ 121 , 122 ], and menstrual cycle [ 122 – 125 ] have been shown to modify the onset and/or sensitivity of the sudomotor response (see Table 1 ). However, modifications in the onset and/or sensitivity of regional sweating in relation to body core temperature are not necessarily associated with significant differences in overall whole-body sweat losses during exercise. Two examples of this were noted above, with respect to the impact of sex and chronological age on sweating. Another example is the menstrual cycle: during the luteal phase regional sweating rate is lower at a given body core temperature (increased threshold and decreased slope) [ 122 – 125 ], but there are no differences in whole-body sweating rate across the menstrual cycle phases [ 123 , 126 – 129 ]. Additionally, for trained females their menstrual phase is of little physiological or performance consequence during exercise in the heat [ 103 , 130 ].
Some of the variability in sweating rate can be explained by differences in the structure of sweat glands. For example, with habitual activation, sweat glands show some plasticity in their size and neural/hormonal sensitivity [ 18 , 19 ]. Sato and colleagues have shown that glandular size (volume) can vary by as much as fivefold between individuals [ 9 , 131 ], and there is a significant positive correlation between the size of isolated sweat glands and their methacholine sensitivity and maximal secretory rate [ 131 ] (see Figure 4 ). Sweat gland hypertrophy and increased cholinergic sensitivity have been reported to occur with aerobic training [ 131 ] and heat acclimation [ 38 ] (see Table 1 for more information).
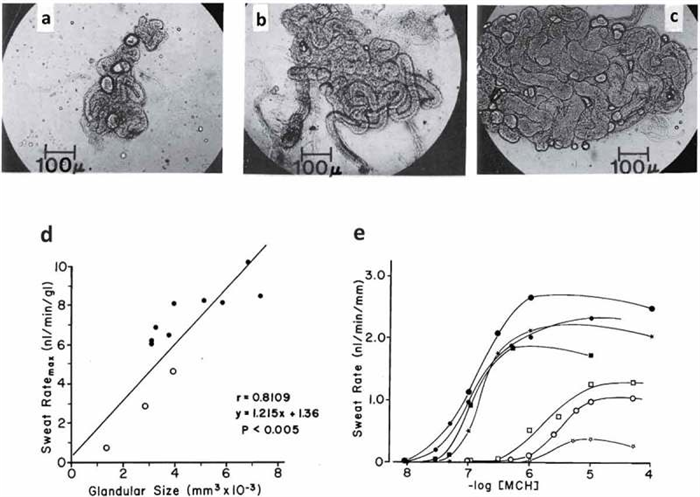
Top row (panels A-C): Variation in the size of human eccrine sweat glands taken from the backs of three different men who were described as poor (A), moderate (B), and heavy sweaters (C). Bottom row: Correlation between size of sweat gland and sweat ratemax per gland (panel D). Dose-response curves (expressed per unit length of tubule) of sweat rates of 7 men to methacholine. Closed symbols show moderate to heavy sweaters. Open symbols show poor sweaters. Reprinted from Sato and Sato 1983 [ 131 ]with permission.
Eccrine sweat composition
Methodological considerations
In science, the accuracy and reliability of study methodology are critical to interpret results and draw conclusions about the impact of an intervention or other factor on the outcome measure of interest. Measuring sweat composition is no exception. Previous papers have comprehensively reviewed the effect of methodology on intra- and inter-individual variability in sweating rate and sweat composition as well as made suggestions for best practices [ 16 , 83 , 132 ]. Therefore, this topic will not be reviewed extensively here. Instead, these methodological considerations and supporting references have been summarized in Table 2 . This table includes the main aspects of sweat methodology that are important to consider when interpreting and designing sweat composition research; these include 1) skin cleaning/preparation, 2) sweat stimulation, 3) sweat collection, 4) sample storage, and 5) analytical technique.
Specific examples illustrating the importance of valid methodology in interpreting study results are discussed throughout the remaining sections of this paper. However, it is worth noting a couple of overarching themes. First, it is important to realize that depending upon the methodology used, sweat collected from the surface of the skin may contain, not only thermal sweat secreted by the eccrine sweat gland but also, residual contents of the sweat duct, sebum secretions, epidermal cells, and other skin surface contaminants. This can lead to artificial elevations in sweat constituent concentrations and, in some cases, the overestimation is not small. For example, as shown in Table 2 , two- to five-fold increases in constituent concentrations have been reported for trace minerals such as Fe and Ca. Unless care is taken to avoid contaminants it is difficult to draw conclusions about sweat composition and its utility as a biomarker, its impact on micronutrient balance, and assess the effectiveness of the sweat gland in excretion of waste products or toxicants. By contrast, dermal contamination from extra-sweat NaCl seems to be negligible compared with NaCl contained in the sweat itself, as studies have reported only 0.2 mmol/h of Cl on the skin without sweat activity [ 133 – 135 ].
Another important methodological consideration is to ensure that the conditions of the protocol, including the method of sweat stimulation and anatomical location of sweat collection, are specific to the research question of interest. As described in Table 2 sudomotor responses vary among pharmacological-, passive heat-, and exercise-induced sweating and so these methods should not be used interchangeably. Similarly, because of regional variability in sweating rate and sweat constituent concentrations, data from one region cannot be generalized to other regions or the whole body.
Overview of sweat composition
Sweat is a very complex aqueous mixture of chemicals. Although sweat is mostly water and NaCl, it also contains a multitude of other solutes in varying concentrations [ 6 , 136 – 139 ]. Tables 3 and 4 list some of the micronutrients and non-micronutrients, respectively, present in sweat. This is obviously not an exhaustive list but includes some of the more commonly researched constituents. Tables 3 and 4 include the range in sweat constituent concentrations, mechanisms of secretion and reabsorption, and functional role in health, where known or applicable. Micronutrients include the electrolytes Na and Cl, which are the constituents found in the highest concentrations in sweat, as well as K, vitamins, and trace minerals. Non-micronutrient ingredients listed in Table 4 include products of metabolism, proteins, amino acids, and toxicants. It is important to note that concentrations listed in these tables are approximate ranges and are not intended to reflect normal reference ranges. There are insufficient data, perhaps with the exception of Na, Cl, and K, to inform normative ranges for sweat constituents at this time. Instead the ranges listed are meant to provide some context in terms of relative order of magnitude of concentrations across all of the constituents, in order of higher (e.g. NaCl) to lower (e.g. trace minerals and heavy metals) concentrations (in mmol/L). For some constituents, higher values outside the range listed have been reported, but are relatively rare, involve individuals with medical conditions, or may be inflated because of methodological issues; all of these points are discussed in more detail in later sections of this paper.
Because interstitial fluid is the precursor fluid for primary sweat, it follows that many components of final sweat originate from this fluid space. However, the exact mechanisms of secretion are largely unknown for most constituents other than Na and Cl. Potential mechanisms and supporting references are listed in Tables 3 and 4 and may include active or passive (diffusion across membranes or paracellular transport) mechanisms of transport. Some sweat constituents do not originate from the interstitial fluid, but instead, appear in sweat as a result of sweat gland metabolism (e.g. lactate) [ 140 ]. Yet others (e.g. antimicrobial peptides, proteolytic enzymes) are thought to be produced by the sweat gland and play a functional role in skin health ( Table 4 ). It should be noted that many other chemicals (not in Tables 3 and 4 ), such as cortisol [ 141 , 142 ] neuropeptides, bradykinin, cyclic AMP, angiotensins, and histamines [ 9 , 15 ] are also present in sweat. Some researchers have hypothesized that one or more of these ingredients may be biologically functional, and involved in the regulation of sweat gland and/or ductal function; however, support for this notion is currently limited [ 9 ]. For a more comprehensive list of sweat constituents, the reader is referred to other published reviews [ 6 , 134 ] and studies [ 139 , 143 ], including metabolomic analysis of sweat [ 136 – 138 ].
Sodium chloride
It is well established that sweat [Na] and [Cl] can vary considerably among individuals. Regional sweat [Na] typically ranges from 10 to 90 mmol/L ( Figure 5(a ); see also [ 144 – 148 ]), while whole-body sweat [Na] is ~20–80 mmol/L (predicted shown in Figure 5(b ); measured in references [ 144 , 146 , 147 , 149 ]). The range in sweat [Cl] is similar, but perhaps slightly lower than that of sweat [Na], with whole-body values reported to be ~20–70 mmol/L [ 134 , 144 , 149 ]. Table 1 shows the host and environmental factors that account for some of the variability in sweat [Na] and [Cl]. The [Na] and [Cl] of final sweat are determined predominately by the rate of Na reabsorption in the duct relative to the rate of Na secretion in the clear cells.
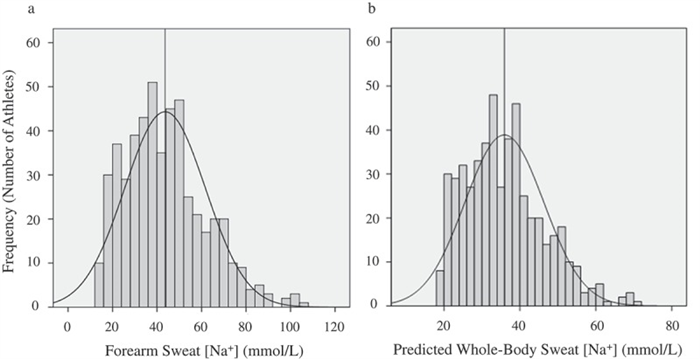
Frequency histograms of forearm sweat sodium concentration (Panel A) and predicted whole-body sweat sodium concentration (Panel B) in 506 skill-sport and endurance athletes during training/competition in a wide range of environmental conditions. The vertical line represents the mean value. Reprinted from Baker et al. 2016 [ 156 ] with permission.
Na ion reabsorption is controlled by Na-K-ATPase activity, which is influenced by plasma aldosterone concentration and/or sweat gland sensitivity to aldosterone. Resting (genomic) plasma aldosterone is dictated by an individual’s chronic physiological condition, dictated in part by heat acclimation, fitness, and diet. Circulating aldosterone also changes acutely in response to non-genomic factors such as exercise and dehydration. Yoshida et al. [ 150 ] demonstrated that individual variations in the sweat [Na] response to an increase in the sweating rate during exercise were correlated with resting aldosterone, but not to exercising aldosterone. Therefore the genomic action of aldosterone may have a stronger impact on inter-individual variations in sweat [Na] than the rapid non-genomic action of aldosterone during exercise in humans [ 150 ]. Both [Na] and [Cl] in sweat are influenced by the availability of CFTR chloride channels; with lower CFTR abundance resulting in less ductal reabsorption and therefore higher final sweat [Na] and [Cl]. CFTR availability is clearly reduced with defects in CFTR genes (i.e. cystic fibrosis, discussed in more detail in the Sweat composition as a biomarker section below) and recent evidence suggests that healthy (non-CF) individuals with salty sweat may also exhibit lower abundance of sweat duct Cl channel CFTR [ 151 ].
Effect of sweat flow rate
Sodium chloride
Sweat flow rate is another important factor determining final sweat [Na] and [Cl] and of other aspects of sweat composition. This concept has been known since as early as 1911 [ 152 ] and several studies since then have confirmed a direct relation between sweating rate and final sweat [Na] and [Cl] [ 5 , 6 , 11 , 15 , 40 , 153 , 154 ]. In 2008, Buono et al. [ 39 ] reported data providing insight to the physiological mechanism responsible for the relation between sweat flow rate and sweat [Na] and [Cl]. They found that as forearm sweating rate increased (from ~0.25 to 0.82 mg/cm 2 /min), the rate of Na secretion in primary sweat increased proportionally more than the rate of Na reabsorption along the duct [ 39 ]. Within this range in sweating rate, which was stimulated via a progressive increase in exercise intensity (from 50% to 90% HRmax), sweat [Na] increased from 19 ± 5 to 59 ± 10 mmol/L ( Figure 6 ) [ 39 ]. An important point is that the absolute rate of Na reabsorption actually increased continuously with increases in sweating rate. However, the percentage of secreted Na that was reabsorbed in the duct decreased with a rise in sweating rate. That is, at the lowest sweating rate 86 ± 3% of the secreted Na was reabsorbed, while at the highest sweating rate only 65 ± 6% of Na was reabsorbed from the duct. Therefore, the faster the primary sweat travels along the duct the smaller the percentage of Na that can be reabsorbed [ 39 ]. Underlying mechanisms are unclear, but Buono et al. [ 39 ] speculated that possible factors could include decreased contact time of sweat with the apical membrane of the duct, saturation of transporters, and/or decreased activity of epithelial sodium channels due to decreased cytosolic pH associated with higher sweating rates. According to some studies, there may be a minimum threshold sweating rate (~0.3 mg/cm2/min) required before sweat [Na] starts to rise with an increase in sweating rate [ 15 , 155 ]. For context, this equates to ~0.3 L/h (for a 1.8 m 2 individual), which is at the very low end of the range of sweating rates expected during exercise/heat stress [ 105 , 156 , 157 ].
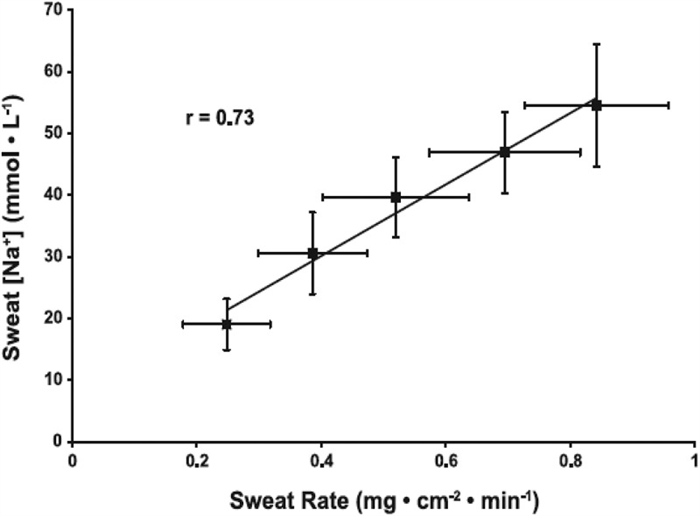
Relation between regional sweating rate and regional sweat [Na]. Values are means ± SE for 10 subjects’ regional (forearm) sweating rate and sweat [Na] while exercising at 50%, 60%, 70%, 80%, and 90% of maximal heart rate. The mean r for the group was 0.73 (P < 0.05). y = 59.7(x)+6.7. Reprinted from Buono et al. 2008 [ 39 ] with permission.
Given the well-established relation between sweat flow rate and sweat electrolyte concentrations, it follows that any factors stimulating acute increases in sweating rate (e.g. increases in air temperature or exercise intensity) within an individual would result in higher sweat [Na] and [Cl] [ 152 , 158 ]. This has been found at the whole-body level [ 159 ] ( Figure 7 ) as well as within isolated sweat glands [ 6 ] and given skin regions [ 39 ]. The effect of sweat flow rate on relative Na reabsorption may also partially explain regional differences in sweat [Na] and [Cl] within subjects. Studies measuring sweating rate and sweat [Na] and/or [Cl] across multiple body sites have found that sites with higher sweating rate also tend to exhibit higher sweat [Na] and [Cl] [ 146 , 147 ]. This concept is illustrated in Figure 8 , which shows a significant correlation (r = 0.71, p < 0.05) between mean regional sweating rate and mean sweat [Na] across eight different regions, where sweat [Na] ranged from 36 mmol/L on the calf (lowest sweating rate) to 72 mmol/L on the scapula (highest sweating rate) [ 149 ].
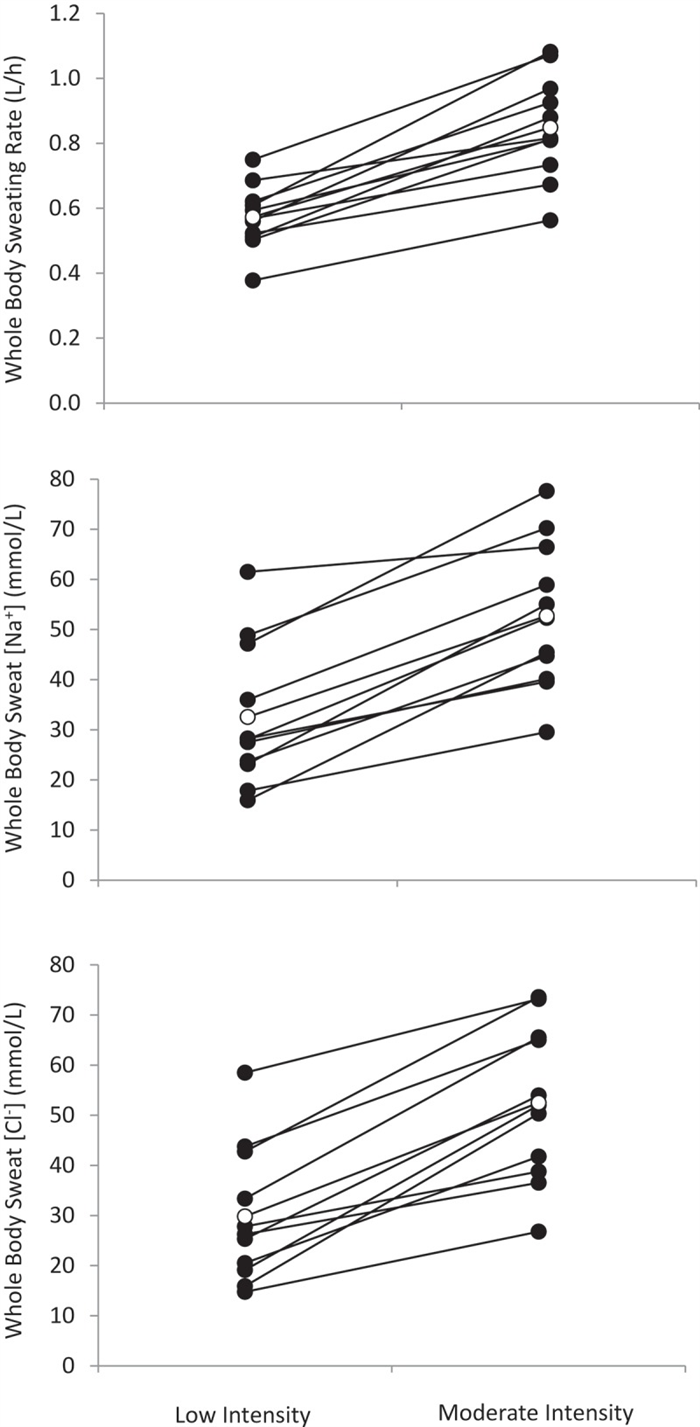
Whole-body sweating rate and whole-body sweat [Na] and [Cl] comparison between low (45% maximal oxygen uptake) and moderate (65% maximal oxygen uptake) intensity cycling exercise in a warm (30°C and 44% relative humidity) environment (n = 11 men and women). Solid circles show individual data. Open circles show mean data (p < 0.05 between low and moderate intensity for sweating rate, sweat [Na] and sweat [Cl]). Redrawn from Baker et al. 2019 [ 159 ].
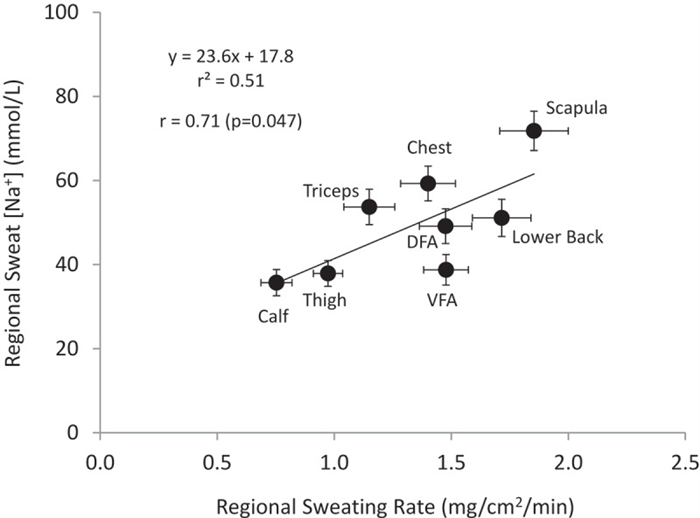
Regional sweating rate vs. regional sweat [Na]. Data points represent the group (26 subjects) mean ± SEM at each regional site (DFA, dorsal forearm; VFA, ventral forearm). Regional sweating rate and sweat [Na] measured with the absorbent patch technique during cycling exercise in the heat (30°C, 42% relative humidity). Redrawn from Baker et al. 2018 [ 149 ].
To date, the relation between sweat flow rate and sweat [Na] has been well-established in studies in which subjects served as their own control (e.g. Figure 6 – 8 ). However, the regional sweating rate vs. regional sweat [Na] relation for between-subject comparisons has been researched to a lesser extent. When plotting regional sweating rate vs. regional sweat [Na] across subjects, Baker et al. [ 149 ] found a significant relation between sweating rate and sweat [Na] at only one region (thigh, r = 0.43) out of 11 regions studied and no significance at the whole-body level ( Figure 9 ). This may suggest that other factors affecting sweat [Na] and [Cl], such as CFTR genes or genomic effects of aldosterone on Na-K-ATPase may play a more important role in determining inter-individual differences in sweat [Na] and [Cl] during exercise/heat stress. On the other hand, acute changes in sweating rate play a significant role in intra-individual differences in sweat [Na] and [Cl] [ 39 , 159 ].

Regression of regional sweating rate vs. regional sweat [Na] within site for the dorsal forearm (A), and the 9-site aggregate (weighted for body surface area and regional sweating rate), as well as regression of whole-body sweating rate vs. whole-body sweat [Na]. Correlations between sweating rate and sweat [Na] were not significant (p > 0.05). Reprinted from Baker et al. 2018 [ 149 ] with permission.
Bicarbonate, pH, and lactate
In addition to Na and Cl conservation, another important function of the sweat gland is reabsorption of bicarbonate for the maintenance of acid-base balance of the blood [ 8 ]. Exact mechanisms are not fully understood, but it is thought that bicarbonate is reabsorbed directly via CFTR chloride channels [ 36 ] and/or hydrogen ions are secreted in the sweat duct [ 5 ]. In the process, sweat fluid in the ductal lumen is acidified before excretion onto the skin surface [ 36 ]. The pH of primary sweat starts at ~7.1–7.4 [ 5 , 8 ]. Bicarbonate reabsorption in the duct is inversely related to sweating rate [ 5 , 8 , 37 , 160 ]. At low sweating rates, the luminal fluid is exposed to the duct for a longer period of time and is acidified to a greater extent, resulting in a decrease in pH to as low as ~4–5 [ 5 , 134 ]. At faster sweat flow rates, pH of sweat can remain as high as ~6.9 [ 5 , 134 ].
As discussed previously, lactate is produced by eccrine sweat gland metabolism [ 13 , 15 , 45 , 134 , 140 ]. Thus, there is a direct relation between sweating rate and lactate excretion rate, such that the higher the sweating rate (and the greater metabolic activity of the sweat gland) the more lactate is secreted in sweat in terms of mmol/min. However, because of the diluting effect of higher sweat fluid volume, there is an inverse relation between sweating rate and sweat lactate concentration [ 161 – 163 ]. Accordingly, sweat lactate concentration decreases with increasing exercise intensity [ 161 , 162 ]. More details regarding the effect of sweat flow rate on sweat composition are provided in Tables 3 and 4 .
Sweat composition as a biomarker
There has been considerable interest recently in the use of sweat as a non-invasive alternative to blood analysis to provide insights to human physiology, health, and performance. The development of wearable devices and sensing techniques for sweat diagnostics is an expanding field. Perhaps the best example of a sweat biomarker is the use of sweat [Cl] for the diagnosis of cystic fibrosis, although this practice is not new [ 164 ]. The association between high sweat Cl and cystic fibrosis was first recognized by di Sant’Agnese et al. in 1953 [ 165 ]; and subsequently, a standardized sweat test (Quantitative Pilocarpine Iontophoretic Test) was developed by Gibson and Cooke in 1959 [ 166 ]. Individuals with cystic fibrosis have higher than normal sweat [Cl] because of a genetic absence of a functioning CFTR (two defective genes, homozygote) [ 167 – 170 ]. The cutoff for a positive sweat test consistent with cystic fibrosis is sweat [Cl] >60 mmol/L [ 171 ]. However, sweat [Cl] in cystic fibrosis patients can be much higher, with values in the 80–130 range commonly reported [ 165 , 166 , 172 – 176 ]. Because the epithelial Na channels depend upon a functioning CFTR, Na is also poorly reabsorbed in individuals with cystic fibrosis [ 170 ]. Individuals with one defective gene for CFTR (heterozygote) may also have elevated sweat [Na] and [Cl] [ 169 , 177 ]. For more details, the reader is referred to the following reviews on cystic fibrosis [ 169 , 178 – 180 ].
Apart from the use of sweat [Cl] for the diagnosis of cystic fibrosis, the application of sweat diagnostics has been limited to date [ 181 , 182 ]. There are perhaps a few constituents in sweat whose concentrations may change in accordance with large disturbances in homeostasis. For example, sweat glucose concentration has been shown to increase 2–3x in response to oral and intravenous glucose which increased blood glucose concentration to 200–250 mg/dl [ 183 ]. In addition, iron-deficient anemic patients have lower than normal [Fe] in sweat (especially in cell-rich sweat [ 184 ]) and sweat [Fe] has been shown to increase with iron therapy [ 185 ]. However, the utility of glucose, micronutrients, and other constituents as sweat biomarkers is questionable, especially as a real-time monitoring tool, because correlations between sweat and blood have not been established. As shown in Tables 3 and 4 , the literature has reported mixed results regarding the correlation between sweat and blood for glucose, cytokines, urea, ammonia, and bicarbonate and no significant correlation for micronutrients, lactate, heavy metals, or environmental toxicants.
One of the proposed uses of sweat composition as a biomarker is the prediction of hydration status from sweat electrolyte concentrations or some ratio of [Na], [Cl], and/or [K] [ 186 – 189 ]. However, a fundamental issue with this assertion is that sweat [Na] and [Cl] are known to vary considerably within and among individuals; and a change in hydration status is only one of many factors that could play a role in this variability [ 132 ]. This is further complicated by the fact that dehydration could have differential effects on sweat [Na] and [Cl]. Dehydration-induced hemoconcentration would increase extracellular [Na] and in turn increase Na of the primary sweat, in theory leading to a small increase in final sweat [Na]. On the other hand, dehydration would also be expected to reduce sweating rate ( Figure 3 ), which would, in turn, lead to lower sweat [Na]. Other factors such as heat acclimatization, exercise intensity, environment, diet, and sweat stimulation/collection methodology influence sweat [Na] and [Cl] ( Table 1 ). These confounding factors likely explain the discrepancy in results across studies measuring sweat composition and changes in hydration status. Dehydration has been associated with increased [ 134 , 153 , 190 ], decreased [ 191 , 192 ], or no change [ 193 – 195 ] in sweat [Na] and [Cl]. Sweat [K] and pH are also poor indicators of hydration status [ 190 ]. Additionally, sweat composition (Na, Cl, K, pH, lactate) explains very little of the variation in individuals’ sweating rate during exercise [ 147 , 149 ].
In summary, while the notion of a non-invasive tool for real-time hydration, nutrition, and health monitoring is attractive, more research is needed to determine the utility of sweat composition as a biomarker for human physiological status. To date, few well-designed, adequately powered studies have investigated the correlation between sweat and blood solute concentrations. Moreover, as discussed throughout this paper, final sweat composition is not only influenced by blood solute concentrations, but also the method of sweat stimulation (active vs. passive), ion secretion and/or reabsorption in the proximal duct, sweat flow rate, byproducts of sweat gland metabolism, skin surface contamination from epidermal cells as well as sebum secretions, among other factors. These challenges need to be considered in future research and applications of sweat diagnostics.
Physiological purpose of sweating: Roles in the maintenance/disturbance of human health
Thermoregulation
It is well-established that the primary physiological function of sweating is heat dissipation for body temperature regulation. The mechanical efficiency of humans is ≤30% [ 196 ]; therefore, during exercise, a large amount of heat is produced by the contracting muscles as a byproduct of metabolism. In addition, heat is transferred from the air to the body when ambient temperature is greater than skin temperature. With sweating, heat is transferred from the body to water on the surface of the skin. The latent heat of vaporization of sweat is 580 kcal of heat per 1 kg of evaporated sweat (2426 J per gram of sweat) [ 197 ]. According to heat-balance theory, the amount of sweat production is determined by the relation between the evaporative requirement for heat balance (Ereq) and maximum evaporative capacity of the environment [ 198 , 199 ]. Ereq is represented by the following equation [ 200 ]:
where M is metabolic energy expenditure, W is external work, R is radiant heat exchange, C is convective heat exchange, and K is conductive heat exchange [ 201 , 202 ]. The primary means by which the body gains heat is from metabolism (which is directly proportional to exercise intensity) and the environment; therefore, these factors are also the primary determinants of sudomotor activity [ 201 , 203 ]. It is important to note that some sweat can drip from the body and not be evaporated. Therefore during conditions of low sweat efficiency (e.g. humid environment), a higher sweating rate than calculated from Ereq may be needed to achieve a given level of evaporation [ 198 , 204 ]. For a more comprehensive discussion on the role of sweat evaporation in human thermoregulation, the reader is referred to other reviews [ 83 , 200 , 205 ].
Skin health
Eccrine sweat is thought to play a role in epidermal barrier homeostasis through its delivery of water, natural moisturizing factors, and antimicrobial peptides to the skin surface. Natural moisturizing factors include amino acids (or their derivatives), lactate, urea, Na, and K; which can act as humectants allowing the outermost layers of the stratum corneum to remain hydrated [ 206 ]. Some of these natural moisturizing factors, such as lactate, urea, Na, and K originate from eccrine sweat [ 206 ], while amino acids on the skin surface may be produced in the stratum corneum [ 207 ]. Nevertheless studies have shown that perspiration increases stratum corneum hydration [ 206 , 208 ] and this may occur via moisture transfer from the eccrine gland coil directly into the skin before commencement of surface sweating [ 13 , 208 ]. Therefore, it has been proposed that preservation of sweating may be an important therapeutic strategy for improving atopic dermatitis or other conditions of dry skin [ 206 , 209 ], albeit direct evidence is still needed. On a related topic, wetting of the skin with eccrine sweat on the palmar surfaces can improve tactile sense and enhance grip as an aspect of the fight or flight response in humans [ 210 ]. Finally, recent immunohistochemistry studies suggest that sweat glands produce and excrete antimicrobial peptides such as dermcidin [ 211 ], cathelicidin [ 212 ], and lactoferrin [ 213 ], pointing to a potential role of sweating in host defense against skin infection [ 214 ]. The reader is referred to recent reviews for more details on the role of sweat in skin hydration [ 209 , 214 , 215 ] and microbial defense [ 216 ].
Role in micronutrient balance
Sweat gland adjustments in response to deficiency or excess
Heat acclimation
Sodium chloride
The changes in sweat [Na] and [Cl] during heat acclimation have been well established and reviewed in previous papers [ 134 , 169 ] and therefore will not be comprehensively discussed here. In brief, adaptation to the heat leads to improved salt conservation through a decrease in sweat [Na] and [Cl] [ 62 , 63 , 152 , 158 , 217 – 220 ]. While the degree of conservation varies across studies due in part to methodological differences, the reported decrease in sweat [Na] and [Cl] after ~10 days of heat acclimation ranges from ~30% to 60%. Most studies have involved a 7–10-day heat acclimation protocol, but Buono et al. [ 217 ] recently showed that Na conservation may begin after just two consecutive days of heat exposure and sweat [Na] decreases linearly over time.
Somewhat paradoxically, the decrease in sweat [Na] and [Cl] occurs despite increases in sweating rate that accompany heat acclimation. This can be explained by the disparate effects of acute changes in sweat flow rate (discussed above) versus the longer-term adaptations in the sweat gland that occur with heat acclimation. Buono et al. [ 218 ] found that the linear relation between sweat flow rate (up to 1 mg/cm 2 /min) and sweat [Na] persist after a 10-day heat acclimation protocol, but there is a downward shift such that the y-intercept of the relation decreased by 15 mmol/L. The slope of the relation did not change after heat acclimation. Thus, at any given sweating rate on the forearm, heat acclimation resulted in significantly lower forearm sweat [Na] [ 218 ]. However, changes in the slope and y-intercept in response to heat acclimation have not been established for the relation between whole-body sweating rate and whole-body sweat [Na] or [Cl]. Most heat acclimation studies have measured regional sweat electrolyte concentrations. Because of the variable effects of heat acclimation on regional sweating rate, such that regional sweating rate on the limbs (forearm) tend to increase proportionally more than at central sites (chest, back) [ 221 , 222 ], future research is needed to confirm the effects of heat acclimation on whole-body sweat [Na] and [Cl] and its relation with whole-body sweating rate.
The underlying mechanism for NaCl conservation is thought to be related to increased sensitivity of the sweat gland to circulating aldosterone [ 62 ]. Aldosterone impacts Na reabsorption in the eccrine sweat duct by increasing Na-K-ATPase activity [ 38 , 223 ]. However, it is important to clarify that the presence of a salt deficit is required for NaCl conservation to occur with heat acclimation. In studies where subjects consumed enough NaCl to replace losses incurred during the repeated exercise-heat stress, sweat [Na] and [Cl] did not change or increased slightly [ 45 , 169 , 224 , 225 ]. This topic will be discussed further in the Diet – Sodium Chloride section below.
Trace minerals
A common question on the topic of heat acclimation is whether or not electrolytes or minerals other than NaCl are conserved. Only a few studies have investigated this and mixed results have been reported. For example, in a study with college basketball players, dermal Ca losses measured via a cotton shirt method decreased by 32% from the first to last day of a 10-day training session [ 226 ]. In 2008, Chinevere et al. [ 219 ] measured sweat mineral concentrations (Ca, Mg, Fe, Zn, and Cu) using the polyethylene arm glove technique and found a 23–75% decrease in mineral concentrations from day 1 to 10 of a heat acclimation protocol. However, in a subsequent heat acclimation study from the same laboratory, Ely et al. [ 227 ] found that the changes in sweat mineral concentrations varied depending upon the sweat collection methodology. In arm bag sweat, [Ca], [Mg], and [Cu] trended progressively downward by 26–29% from day 1 to 10 [ 227 ]. However, there were no changes in sweat mineral concentrations with heat acclimation at the scapular site that had been thoroughly washed [ 227 ]. The authors attributed the decline in sweat mineral concentrations in this [ 227 ] and their previous heat acclimation study [ 219 ] to an artifact of epidermal contamination when using the arm bag technique and/or not pre-washing/cleaning the skin at the site of collection [ 228 ]. That is, progressive flushing of mineral residue lying on the skin surface with daily-repeated profuse sweating may have contributed to the decrease in sweat mineral concentrations over the 10 days of testing [ 227 ].
There have been some suggestions that conservation of sweat trace mineral loss occurs on an acute basis during a single bout of exercise. For example, several studies have shown decreases in sweat mineral (Fe, Zn, Mg, Ca) concentrations during 1–7 h of exercise [ 229 – 232 ]. Because sweat mineral concentrations decreased despite stable or increasing sweating rates over time, it was hypothesized that mineral conservation may have been taking place. However, again, this is likely an artifact of skin surface contamination, as studies using methodology to collect clean or cell-free sweat have shown no evidence of trace mineral conservation in response to acute exercise-induced sweating [ 228 , 233 , 234 ]. Moreover, there are no known physiological mechanisms by which Ca, Mg, Fe, Cu, and other trace minerals would be reabsorbed by the eccrine sweat gland duct in order to facilitate conservation of loss via sweating.
Diet
Sodium chloride
It is a common perception that Na ingestion influences sweat [Na] or the rate of sweat Na excretion. However, study results to date have been mixed. For example, in a systematic review of six endurance exercise studies, McCubbin and Costa (2018) found no relation between the change in Na intake and the change in sweat [Na] across studies. For example, in one study Costa et al. [ 235 ] found just a 4 mmol/L mean difference in whole-body sweat [Na] in men after 6-weeks of consuming either 3.4 g Na/day or 5.6 g Na/day (2.2 g/day intake difference). On the other hand, Hargreaves et al. [ 236 ] reported a 12 mmol/L difference in whole-body sweat [Na] after 2 weeks of either 1.15 g Na/day or 3.45 g Na/day (2.3 g/day intake difference). Thus, McCubbin and Costa concluded that the impact of dietary Na intake on sweat [Na] during exercise is uncertain and future studies are needed [ 237 ].
Table 5 shows a summary of the studies assessing the effects of Na intake on sweat electrolyte concentration and total sweat electrolyte loss during exercise and/or heat stress. The disparate results among studies may be reconciled in part by considering the time course of sweat glands’ response to variations in salt balance and associated changes in circulating aldosterone. As noted by Robinson in the early 1950s, while the renal system responds to a salt deficiency or excess within 1–3 h, the sweat glands typically require 1–4 days [ 134 , 238 ]. The literature summary in Table 5 is in general agreement with this notion. Indeed, most studies have shown that several days to weeks of dietary Na manipulation are associated with changes in sweat [Na] [ 45 , 224 , 225 , 235 , 236 , 239 – 241 ]. Other studies, usually of shorter duration (up to 3 days) [ 195 , 242 ] or with relatively small changes in daily Na ingestion [ 243 , 244 ] have reported no or minimal effect of dietary Na on sweat [Na] or the rate of Na excretion. The relation between acute (i.e. shortly before/during exercise) Na intake and sweat [Na] has not been well-studied. However, in one investigation, Hamouti et al. (2012) found no differences in sweat [Na] when various amounts of Na (0, 1.45 g, or 2.9 g) were ingested 1.5 h before exercise [ 245 ]. This result is perhaps not surprising based on the time course of sweat gland responsiveness, which is also in agreement with the notion that genomic effects of aldosterone on sweat [Na] are stronger than non-genomic actions (as discussed above in the Overview of Sweat Composition section) [ 150 ]. Regardless of duration, all studies have been consistent in finding no effect of salt deficiency or excess on sweating rate ( Table 5 ). Therefore, any change in the rate of sweat NaCl excretion associated with dietary NaCl is likely due to changes in sweat concentrations.
Finally, it is important to discuss the dietary Na vs. sweat [Na] literature within a practical context. Several studies have employed study designs with large, perhaps unrealistic changes in dietary Na intake. For example, in five out of the 12 studies in Table 5 the high Na diet consisted of ≥8 g Na/day sustained over 5 days or more [ 45 , 224 , 240 – 242 ]. The low Na diet in these same studies was 0.5 to 2.3 g/day, resulting in vast differences in controlled daily Na intakes (by at least ~6 g). Few studies included “normal” dietary Na trials, which is 3.4 g/day for Americans [ 246 ]. The variation in sweat [Na] as a result of smaller deviations in Na intake, more realistic to a free-living individual, is yet to be fully elucidated. Nonetheless, in these five studies, the change in mean sweat [Na] was inconsistent, ranging from −5 mmol/L to +30 mmol/L. In addition, some studies measured sweat [Na] via regional techniques [ 240 , 243 ], which may not be indicative of changes at the whole-body level. Others have used a parallel study design where sweat [Na] was not matched between groups at baseline [ 241 ]. Thus, it is important that future studies address these and other methodological limitations as also pointed out by McCubbin and Costa [ 247 ].
Trace minerals
Several studies have investigated the hypothesis that dietary intake of trace minerals and vitamins influences sweat composition. However, most [ 230 , 231 , 248 – 251 ] but not all [ 184 , 185 , 252 ] studies reported no association between dietary intake of trace minerals (Zn, Fe, Ca, Cu) and their concentrations or excretion rates in sweat. This research has included acute supplementation (within ~24 h of sweat collection) as well as controlled and free-living chronic dietary intake of minerals and vitamins. Regardless of study duration, the impact of diet on sweat mineral and vitamin loss seems to be minimal, at least in healthy individuals with no known deficiencies. For example, Vellar et al. [ 251 ] measured whole-body cell-free sweat before and after giving an acute oral iron load (ferrous succinate tablets) to healthy men that led to nearly a twofold increase in serum [Fe]. There was no change in sweat [Fe] or sweating rate during 60 min of passive heat stress as a result of the acute iron load [ 251 ]. Similarly, Lug and Ellis [ 250 ] found no significant changes in sweat vitamin concentrations in healthy heat-acclimatized men after administration of a dietary supplement of 500 mg L-ascorbic acid during the 24 h before sweat collection. Furthermore, in a 30-day controlled diet study in healthy men, Jacob et al. [ 248 ] found no association between dietary intake of Zn, Cu, and Fe and whole-body sweat [Zn], [Cu], and [Fe], respectively.
A few studies have found a significant change in sweat mineral concentrations associated with dietary intake [ 184 , 185 , 252 ] and the commonality of these studies is that they included patient populations with known mineral deficiencies or involved controlled interventions designed to deplete and subsequently replete mineral stores of healthy subjects. For example, Milne et al. [ 252 ] measured daily whole-body sweat Zn loss during controlled periods of Zn intake. For the first 5 weeks, Zn intake was 8.3 mg/d, then reduced to 3.6 mg/d for the next 16 weeks, followed by an increase to 33.7 mg/d for the final 4 weeks of the study. Corresponding sweat Zn loss was 0.49 mg/d, 0.24 mg/d, and 0.62 mg/d, respectively; equivalent to a 51% decrease with Zn restriction and a 27% increase with excess dietary Zn [ 252 ]. It is also important to interpret these results within the context of the source of mineral concentrations found in sweat. As pointed out by Milne et al., [ 252 ] sweat samples included desquamated cell debris as well as cell-free eccrine sweat. Therefore, in this study [ 252 ] it is difficult to discern how much of the sweat Zn originated from the body surface (epidermal cells) versus the interstitial fluid (secreted by the eccrine sweat gland), as changes in body mineral homeostasis can impact the mineral stores of the skin as well as that of the interstitial fluid [ 184 , 185 , 253 ].
Some studies have compared mineral concentrations of cell-free and cell-rich sweat in Fe and Zn-deficient patient populations versus healthy normal controls [ 184 , 185 ]. Prasad et al. [ 184 ] found that [Fe] and [Zn] in cell-rich sweat was lower in patients versus the healthy control group. However, in cell-free sweat, only [Zn] was lower in patients, while there were no differences in [Fe] between Fe and Zn-deficient patients and healthy controls. This study suggests that most of the Fe collected at the skin surface originates from desquamated epithelial cells, while most of the Zn is present in the cell-free portion of sweat. This may also partly explain why an acute increase in blood [Fe] in the study by Vellar et al. [ 251 ] resulted in no change in cell-free sweat [Fe].
There are no known reabsorption or secretion mechanisms by which the eccrine sweat gland could actively conserve or preferentially excrete minerals. Therefore, any significant changes in sweat mineral concentrations would be expected to be a result of significant changes in the mineral content of interstitial fluid (impacting cell-free sweat) and/or epidermal cells (impacting cell-rich sweat). Therefore, sweat mineral concentrations may be altered in situations of depletion in intervention studies or chronic deficiencies in patient populations. Note that this is not necessarily evidence of a homeostatic mechanism; rather a result of passive transport of minerals in accordance with concentration gradients during secretion of primary sweat in the secretory coil (cell-free sweat) and an artifact of surface contamination (cell-rich sweat). Furthermore, the impact of diet on cell-rich and cell-free sweat mineral concentrations will differ depending upon the mineral of interest. As discussed above, Fe and Ca are found in much higher concentrations, and Zn in lower concentrations in cell-rich versus cell-free sweat [ 134 , 184 , 233 , 234 , 254 ]; further complicating the interpretation of study results. Future studies on diet, mineral balance, and sweat mineral losses should carefully choose the methodology employed and consider the source of the minerals measured in the sweat. Regardless, based on the available evidence to date, the take-home message for healthy individuals is that small fluctuations in dietary mineral intake (that do not significantly alter mineral status or whole-body stores) seem to have minimal impact on sweat mineral loss.
Sweating-induced deficiencies
Sodium chloride
It is important to define or contextualize high sweat [Na] and [Cl], sometimes referred to as “salty sweat”. The individuals with hyponatremia referenced in the case studies were reported to have sweat [Na] values >80 mmol/L and sweat [Cl] >70 mmol/L as measured on the forearm using the pilocarpine-stimulated sweat test. According to normative data, a forearm sweat [Na] of 80 mmol/L is approximately two standard deviations above average [ 156 ] ( Figure 5 ). “Salty sweat” has been observed in both healthy individuals [ 151 , 263 , 266 , 268 , 269 ] and cystic fibrosis patients [ 174 – 176 , 259 , 263 , 267 , 270 ]. Regardless of the underlying cause of the high sweat [Na] and [Cl], case reports and theoretical models alike demonstrate that excessive electrolyte losses through sweating can contribute to the development of Na and Cl imbalances.
Trace minerals and vitamins
There have been some suggestions that athletes may require dietary supplementation of certain trace minerals due in part to excessive losses in sweat. The two trace minerals that have received the most attention in terms of sweat-induced deficiencies are Ca and Fe. For example, the most recent consensus statement from the International Olympic Committee mentions that excess losses in sweat, in combination with other factors, may lead to suboptimal Fe status in athletes and therefore may require dietary supplementation [ 271 ]. Other papers have suggested that sweat or dermal Ca losses in athletes may contribute to reduced bone mineral density through stimulation of parathyroid hormone during training [ 226 , 272 , 273 ]. However, the balance of the evidence suggests that sweat losses probably contribute minimally to whole-body trace mineral and vitamin deficiencies [ 134 , 148 , 250 , 253 , 274 – 279 ].
First, it is important to reiterate that many of the studies reporting substantial trace mineral and vitamin losses in sweat have used methods (e.g. arm bag or other regional techniques, scraping methods, minimal cleaning, inclusion of initial sweat at start of exercise) [ 226 , 280 – 284 ] that overestimate sweat vitamin and mineral concentrations, including [Ca] and [Fe], by up to 2-3 fold [ 134 , 228 , 233 , 234 ]. For example, 65 years ago Robinson and Robinson [ 134 ] recognized that a primary source of Ca and Fe found in sweat is associated with desquamated cell debris, which is characteristic of the arm bag technique. Regional measures of sweat trace minerals are also higher and more variable (e.g. inter-regional differences) than that of whole body [ 285 , 286 ], which makes it difficult to draw conclusions about the amount of sweat Ca and Fe losses and impact on homeostasis.
Studies have shown that during an acute bout of 1–2 h exercise serum ionized [Ca] decreases, resulting in subsequent elevation of parathyroid hormone and activation of bone reabsorption. The long-term concern with this is a reduction in athletes’ bone mineral density throughout the course of training. While the underlying mechanisms are yet to be elucidated, one hypothesis is that the exercise-induced increase in PTH is triggered by sweat Ca loss. However, only one study has reported an association between sweat Ca loss and any measure of Ca homeostasis or bone mineral density. Barry and Kohrt [ 273 ] found a significant inverse correlation between sweat Ca losses (measured during 2-h cycling) and baseline bone mineral density at the hip, femoral neck, and femoral shaft (r = −0.6 to −0.8) in competitive male cyclists. While the cyclists’ bone mineral density decreased over the course of one year of training, there was no correlation between sweat Ca loss and changes in bone mineral density [ 273 ]. Several other studies have reported no association between sweat Ca loss and measures of Ca homeostasis (bone mineral density, parathyroid hormone, C-terminal telopeptide of Type I collagen, or bone-specific alkaline phosphatase) in female cyclists [ 287 ], male cyclists [ 288 , 289 ], basketball players [ 226 ], or firefighters [ 277 ].
It is important to note that Ca supplementation (or infusion) can attenuate increases in PTH and activation of bone resorption during exercise [ 278 , 287 , 288 ]; however, the underlying mechanism is apparently unrelated to replacement of sweat Ca loss. In addition to the lack of evidence discussed above, the timing of changes in Ca homeostasis during exercise does not agree with the sweat Ca loss hypothesis. As pointed out by Kohrt et al. [ 278 ], because the decrease in serum ionized [Ca] occurs early in exercise (first 15 min) it is unlikely that the extent of Ca loss would be large enough to impact Ca homeostasis. Furthermore, while in extreme circumstances excess mineral loss cannot be ruled out as a contributing factor to suboptimal trace mineral status [ 290 ], for most athletes the main routes of loss are likely through other avenues such as urine or the gastrointestinal tract [ 249 , 291 , 292 ]. Taken together, micronutrient supplementation does not seem to be necessary on the basis of sweat excretion during physical activity, provided that dietary intakes are normal [ 250 ].
Comparison of sweat gland and kidney function
Water conservation and excretion
The sweat glands are often compared to the nephrons of the kidneys, whose main function, among others, is to conserve the vital constituents of the body [ 293 ]. Indeed, sweat glands share some similarities with the renal system; as eccrine glands have mechanisms to conserve Na, Cl, and bicarbonate losses in sweat as discussed in detail in the Mechanisms of secretion and reabsorption section above. For example, in response to aldosterone, sweat glands increase Na reabsorption in the duct leading to a decrease in sweat [Na], albeit with a greater time lag than that of the kidneys. A vital function of the kidneys is to regulate body water balance, stimulating diuresis with overhydration and antidiuresis with hypohydration and/or heat stress. These adjustments are mediated through changes in renal water reabsorption in response to arginine vasopressin (AVP) concentrations in the plasma [ 294 ]. With hyperosmotic hypovolemia, AVP binds to vasopressin type 2 receptors of the distal tubule and collecting duct of the kidneys, stimulating aquaporin transport of water. It has been suggested that AVP might facilitate eccrine gland water reabsorption in a similar manner, resulting in attenuated sweating rates and more concentrated sweat (as a consequence of water removal from the primary fluid along the duct) [ 295 – 298 ]. However, the majority of studies have concluded that neither administration of AVP (e.g. subcutaneous injection of pitressin) nor suppression of its effects (e.g. via ethanol ingestion) alter sweating rate or sweat electrolyte concentrations during heat exposure or exercise [ 193 , 299 – 305 ]. These studies also reported no correlation between plasma AVP concentrations and sweating rate or sweat [Na] [ 151 , 302 , 306 ]. Moreover, one study showed that pharmacological manipulation of vasopressin type 2 receptors with an agonist (desmopressin) or antagonist (tolvaptan) prior to exercise had no effect on sweat [Na] [ 306 ]. These results may be explained in part by the relatively sparse ductal membrane expression of aquaporin-5 compared with the secretory coil [ 151 ]. Taken together it appears that AVP does not regulate water loss via the sweat glands as it does in the kidneys; and the sweat duct does not play an important role in water conservation during exercise-heat stress [ 210 , 301 , 306 , 307 ]. Additionally, a recent study suggests that intradermal administration of atrial natriuretic peptide, a cardiac hormone that promotes urinary excretion of sodium and water, has no effect on sweating rate in young adults nor does it affect sweating in response to muscarinic receptor activation [ 308 ].
Excretion of toxicants
The notion that sweating is a means to accelerate the elimination of persistent environmental contaminants from the human body has been around for many years [ 309 , 310 ]. Detoxification methods include several hours per day of sauna bathing to stimulate excessive sweating, resulting supposedly in purification of the body and release of toxins from the blood. Some proponents of this method claim that increasing sweating via exercise or heat stress (sauna) is an effective clinical tool to protect against or overcome illness and disease [ 311 – 313 ]. Others suggest that physical activity leads to better health outcomes as a result of accelerated toxin elimination via thermal sweating [ 314 , 315 ]. As attractive as this idea sounds, there is little if any evidence to date that supports these claims [ 309 , 310 ].
In a series of studies, Genuis et al. [ 311 – 313 , 316 – 318 ] measured the concentration of environmental toxicants in the blood, sweat, and urine of humans. The overall finding of these studies was that many chemicals, including persistent organic pollutants, heavy metals, bisphenol A (BPA), and phthalate are excreted in sweat. Interestingly, the concentrations in sweat were often higher than that of blood and/or urine, and in some cases, chemicals were detected in sweat but absent in blood and urine. Such reports [ 280 , 314 , 318 ] have led some to hypothesize that these chemicals are perhaps preferentially excreted in sweat to reduce the body burden. However there are several important methodological limitations to consider when measuring environmental toxicants in sweat. First, many of these studies used sweat collection methods that are susceptible to surface contamination and sweat evaporation, which would artificially increase the concentration of toxicants measured in sweat samples. For instance, in most of these studies [ 311 – 314 , 316 – 318 ], sweat was collected by the subjects on their own (uncontrolled, unsupervised), from any site on their body, by scraping sweat from the skin surface with a stainless steel spatula into a glass jar. With these methods, it is probable that sweat samples were tainted with sebum secretions. Scraping methods increase the likelihood of skin surface (epidermal cells) contamination because scraped sweat contains 4-10x more lipid than clean sweat [ 319 ]; potentially explaining the high concentrations of some the of lipophilic toxicants in sweat. Furthermore, the method of sweat stimulation (exercise, sauna) and timing (with respect to how long sweating had commenced before collection) were not controlled [ 311 – 314 , 316 – 318 ]. Other studies [ 280 , 320 ] used the arm bag method which is also susceptible to skin surface contamination. As previously discussed the epidermis contains many contaminants, including heavy metals measured in these studies (arsenic and lead) [ 321 , 322 ].
When using these methods Genuis et al. [ 311 ] found consistently higher concentrations of BPA in sweat than urine. Furthermore, BPA was detected in the sweat of 16 of the 20 subjects, but only two of the 20 subjects had BPA in their serum. In another study, PCB52 concentration was higher in sweat than blood and urine [ 316 ]. Given that interstitial fluid is the precursor to primary sweat secretion it is unlikely that the BPA or PCB52 collected at the skin surface in these studies can be attributed to eccrine sweat if the chemical is absent in the blood. Instead the chemicals could have originated from sebum secretions or epidermal cell contamination. One study lends support for this line of thinking: Porucznik et al. [ 323 ] collected sweat (via PharmChek absorbent patches) and urine for 7 days and found BPA in urine, but an absence of BPA in sweat. For example, the highest measured urine BPA concentration was 195 ng/ml for an individual with deliberate exposure, but no BPA was detected above background in the corresponding sweat patch. These results suggest that the renal system is primarily responsible for BPA elimination from the body and pharmacokinetic studies provide additional support for this: in humans, it has been shown that 84–97% of BPA is eliminated in urine 5–7 h after exposure and 100% is eliminated after 24 h [ 324 ]. The primary avenue for heavy metals excretion, based on tracer studies, is fecal output [ 325 ]. Meanwhile, there are no known mechanisms by which the sweat glands would preferentially secrete (concentrate) BPA, persistent organic pollutants, and trace metals to facilitate transport out of the body. Thus direct evidence for sweating as an effective detoxification method is lacking.
Still, future well-controlled studies designed to collect clean eccrine sweat are needed to clarify or refute any potential role of sweating as a therapeutic tool to eliminate toxins from the body. While therapeutic health benefits (mostly subjective measures) from detoxification protocols in some patient populations have been documented, it is important to note that sauna is only one component of a holistic intervention [ 310 ]. Most protocols also include several weeks of strict changes in diet, exercise, and sleep and therefore it is not possible to attribute any benefit solely to sauna therapy [ 309 , 310 , 326 – 328 ]. Moreover, the sauna protocols used in these studies have employed 2–5 h/day of excessive sweating. The efficacy of lower rates of sweat loss, more realistic to the context of everyday life, is unknown [ 309 ].
Excretion of ethanol
Another perceived function of the eccrine sweat glands is the elimination of ethanol from the body through increases in sweating rate and/or sweat ethanol concentration. In fact, increased sweating is often considered a hangover symptom and is part of the Alcohol Hangover Severity Scale used as the standard in alcohol hangover research [ 329 ]. Furthermore, it is commonly believed that an effective cure for hangovers after heavy drinking is to stimulate sweating (via exercise or sauna bathing) to accelerate recovery from alcohol intoxication. However, the evidence to date does not support these ideas; not to mention there are significant health concerns with sauna bathing during alcohol hangover [ 330 ].
In a validation study, the 12-symptom Alcohol Hangover Severity Scale questionnaire (which includes perceived sweating as well as fatigue, dizziness, clumsiness, thirst, nausea, and others) had a hangover severity predictive validity of r 2 = 0.92 [ 329 ]. Interestingly though, perceived sweating was not significantly different between the hangover and control groups in this naturalistic study, while all other individual symptoms successfully differentiated between the two conditions [ 329 ]. Furthermore, alcohol intake has been found to have no or minimal impact on sweating rate in laboratory intervention studies [ 302 , 331 – 333 ]. For instance, two separate studies found no differences in regional sweating rate (chest or upper arm) in response to hot water immersion [ 332 ] or exercise-heat stress [ 331 ] after alcohol ingestion (that lead to 0.07–0.11 g/dl blood alcohol concentration) compared with the placebo conditions. One study did find a higher chest sweating rate during passive heat stress (33°C) 10–30 min after 0.36 g/kg alcohol ingestion compared with water. However, the elevated sudomotor response was transient, as sweating rate decreased after 30 min and became even with the water trial by 40 min into heating [ 333 ]. In addition, differences in sweating rates were very low (up to 0.1–0.2 mg/cm 2 /min) and unlikely to be of practical significance from a detoxification perspective. On the whole-body level, the difference would be equivalent to ≤100 g over the course of 30 min in a 1.8 m 2 individual, but this is likely to be an overestimate since chest sweating rate is higher than that of whole body. On this point, no study to the author’s knowledge has measured changes in whole-body sweating rate in response to alcohol ingestion, either immediately after ingestion or during alcohol hangover.
It does seem that sweat ethanol concentration increases with ethanol ingestion and rises linearly with increases in blood alcohol concentration. For example, Buono et al. collected serial sweat samples (using the anaerobic technique) via pilocarpine iontophoresis for 3 h after 13 mmol/kg ethanol ingestion and found a significant correlation between blood versus sweat ethanol concentrations and the slope of the relation was 1.01 [ 334 , 335 ]. This nearly identical ethanol concentration between blood and sweat supports the idea that sweat ethanol originates from the interstitial fluid and its concentration is not significantly altered during transport through the duct onto the skin surface; which is counter to the suggestion that the sweat glands have homeostatic mechanisms to detoxify the blood (via concentrating mechanisms). Moreover, the main avenue of ethanol elimination from the body is known to be via oxidation by alcohol dehydrogenase and aldehyde dehydrogenase eventually breaking ethanol down to acetyl CoA, all of which occurs in the liver. It is in this manner that 90% of alcohol is removed from the body, with the other 10% being excreted in breath, sweat, and urine [ 336 ]. Taken together the available evidence suggests that sweating likely plays a very small role in alcohol detoxification or hangover cures.
Excretion of metabolic waste
Another important function of the kidneys is excretion of metabolic and dietary waste products. Since some waste products appear in sweat the eccrine glands are also thought of as an excretory organ. For example, sweat contains urea, the major nitrogen-containing metabolic product of protein catabolism. According to Sato [ 15 ], urea readily crosses the eccrine glandular wall and cell membrane and therefore concentrations of urea in sweat are expected to be about the same as that of the plasma. Some studies report very high urea concentrations in sweat [ 337 – 339 ], up to 50x that of serum [ 337 ], and suggest that this is evidence for a selective transport mechanism across the sweat gland, especially in patients with kidney damage, to clear the blood of high urea concentrations [ 337 ]. However, many of these studies used methods susceptible to sample evaporation (collection of sweat drippage) [ 337 , 339 ] or surface contamination (sweat collected at onset of exercise) [ 338 ], which can lead to artificial increases in sweat urea concentrations (see Table 2 ). Other studies have shown that uric acid and creatinine excretion via sweat is insignificant compared with elimination rates through the kidneys [ 337 , 339 ]. Taken together, there is limited evidence that the sweat glands excretory function makes a substantial contribution to homeostasis [ 134 , 193 ].
Altered sweat gland function from conditions and medications
As shown in Table 6 , certain medical conditions and medications can impact sweating rate and sweat composition. As discussed in the Thermoregulation section above, evaporation of sweat is crucial for temperature regulation in warm conditions and this is evident in patients suffering from anhidroses. Diabetes mellitus, multiple sclerosis, spinal cord injury, and anhidrotic ectodermal dysplasia are associated with a significant reduction in sweating; which, in some cases, can severely impair one’s ability to dissipate heat during higher thermal loads [ 340 – 344 ]. In particular, heat intolerance is well documented in patients with anhidrotic ectodermal dysplasia, a genetic condition resulting in a paucity of sweat glands over the entire body surface [ 3 , 15 ]. In addition, the important function of salt conservation by the sweat gland is evident in patients with reduced ion reabsorptive capacities due to a genetic deficiency or absence of functioning CFTRs (cystic fibrosis) or impaired adrenal cortex function (Addison’s disease) [ 345 , 346 ]; who may be more susceptible to electrolyte imbalances [ 174 – 176 , 259 , 263 , 265 , 267 ].
Table 6.
Conditions and medications that alter sweat gland function.
| Timing | Effect on sweating rate and/or sweat composition | |
|---|---|---|
| Selected conditions and medications | ||
| Cystic fibrosis | Chronic | Higher sweat [Na] and [Cl] than normal because of a genetic deficiency or absence of functioning CFTR leading to lower Na and Cl reabsorption rates in the sweat duct [ 167 ,168,170] |
| Addison’s disease | Chronic | Higher sweat [Na] and [Cl] than normal because of impaired adrenal cortex function (aldosterone secretion) leading to lower Na and Cl reabsorption rates in the sweat duct [ 345 ,346] |
| Diabetes mellitus | Chronic | Reduced sweating with T1DM and T2DM; potential mechanisms related to autonomic neuropathy and reduced thermosensitivity, reduced maximal sweating rate, and/or lower number of active sweat glands; impaired ability to dissipate heat, especially during higher thermal loads and in individuals with lower fitness level [ 340 –344] |
| Multiple sclerosis | Chronic | Reduced sweating because of lesions within central nervous system leading to reduced sweat output per gland [ 459 ] |
| Spinal cord injury | Chronic | Reduced or complete absence of sweating in the insensate skin due to disruption in neural pathways involved in central and peripheral control of sweating [ 460 ,461]; compensatory increase in sweating occurs in sensate skin above the spinal injury [ 462 ,463] |
| Severe burns and skin grafting | Chronic | Reduced or complete absence of sweating in the burned area because entire epidermal and majority of the dermal layer (including sweat glands) are excised. Disruption in sweating remains even as the skin graft heals [ 347 –349] |
| Sunburn | Acute | Reduced sweating in artificially-induced mildly sunburned skin [ 350 ] |
| Miliaria rubra (heat rash or prickly heat) | Acute | Reduced sweating because of pore occlusion via keratin plugs causing mechanical blockage of sweat flow onto skin surface; caused by high humidity (excessive sweat) on skin surface for long duration [ 351 ,352] |
| Atopic dermatitis (eczema) | Episodic | Reduced sweating onto the skin surface because of obstruction of sweat pores by keratin plugs, leakage of sweat into dermal tissue around the glands, and/or potentially histamine-induced sweat suppression [ 215 ,353,354]; sweat glucose concentration may be higher than normal with acute atopic dermatitis [ 464 ] |
| Anhidrotic ectodermal dysplasia | Chronic | Reduced or complete lack of sweating because of genetic paucity or absence of sweat glands over entire body surface [ 3 ] |
| Primary hyperhidrosis | Chronic/Episodic | Increased sweating with focal or bilateral distribution affecting primarily the axilla, palms, soles, and craniofacial areas [ 356 ,359]. Etiology involves neurogenic overactivity of otherwise normal sweat glands [ 3 ,29]; associated with genetic predisposition [ 359 ,465]. Limited data on sweat composition. |
| Secondary hyperhidrosis | Chronic/Episodic | Increased sweating with generalized or unilateral distribution as a result of underlying physiologic condition (fever, pregnancy, menopause), pathology (malignancy, infection, cardiovascular disease, endocrine/metabolic, neurological or psychiatric disorders), or medication [ 3 ,356–359]. Limited data on sweat composition. |
| Tattoos | Chronic | Reduced sweating rate and higher sweat [Na] in response to pharmacologically-induced local sweating than non-tattooed skin; unknown etiology [ 466 –468]. More research involving exercise or heat-induced whole body sweating is needed. |
| Medications | Acute/ Chronic |
Antimuscarinic anticholinergic agents, carbonic anhydrase inhibitors, and tricyclic antidepressants can cause generalized hypohydrosis; cholinesterase inhibitors, SSRI, opioids, and TCA can cause generalized hyperhidrosis [ 346 ,355]. Limited data on sweat composition. |
CFTR: cystic fibrosis transmembrane conductance regulator; SSRI: selective serotonin reuptake inhibitors; T1DM and T2DM, type 1 and 2 diabetes mellitus; TCA: tricyclic antidepressants
Other conditions associated with reduced sweating include burns and skin grafting [ 347 – 349 ], sunburn [ 350 ], miliaria rubra [ 351 , 352 ], and atopic dermatitis [ 215 , 353 , 354 ], as well as medications that interfere with neural sudomotor mechanisms (e.g. anticholinergics and tricyclic antidepressants) [ 346 , 355 ]. Hyperhidrosis, where sweating occurs in excess of thermoregulatory demands, can occur with primary etiology [ 3 , 29 ] or secondary to physiologic condition (fever, pregnancy, menopause), pathology (malignancy, endocrine, metabolic, or psychiatric disorder), or medication (cholinesterase inhibitors, SSRIs, opioids) [ 3 , 356 – 359 ]. However, these types of hypo- and hyperhidrosis are often localized and/or episodic and the impact on whole-body thermoregulation and/or fluid balance during exercise and/or heat stress is not well-studied. The reader is referred to the supporting references in Table 6 for more details on each of the conditions and medications that alter sweat gland function.
Conclusions
This paper discussed sweat gland physiology and the state of the evidence regarding various roles of sweating and sweat composition in human health. Based on this review of the literature, the following conclusions were drawn:
It is well established that eccrine sweat glands have a tremendous capacity to secrete sweat for the liberation of heat during exercise and exposure to hot environments. They also have the capacity to enhance sweating rate with heat acclimation for improved heat tolerance.
Eccrine sweat glands reabsorb NaCl and bicarbonate to minimize disruptions to whole-body electrolyte balance and acid–base balance, respectively.
NaCl reabsorption by the sweat glands improves with whole-body NaCl deficits (heat acclimation, dietary restriction), but the response is somewhat delayed (1–3 days) compared with that of the kidneys (within 1–3 h).
Individuals with salty sweat (e.g. [Cl] and [Na] ≥70–80 mmol/L) have an increased risk of NaCl imbalances during prolonged periods of heavy sweating.
Eccrine gland mechanisms for secretion and reabsorption of other sweat solutes are poorly understood; nonetheless, sweating-induced deficiencies appear to be of minimal risk for trace minerals (e.g. Ca and Fe), vitamins, and other constituents.
Eccrine sweating may play a role in skin hydration and microbial defense, but additional research is required.
The role of the sweat glands in eliminating waste products and toxicants from the body seems to be minor compared with other avenues of breakdown (liver) and excretion (kidneys and gastrointestinal tract).
Evidence for a selective mechanism to excrete metabolic and dietary waste products and toxicants via the sweat glands is lacking. That is, sweat glands do not appear to adapt in any way to increase excretion rates of these substances (either via concentrating sweat or increasing overall sweating rate) as the kidneys do in contributing to the regulation of blood concentrations.
Unlike the renal system, sweat glands do not appear to conserve water loss or concentrate sweat fluid through AVP-mediated water reabsorption.
Studies suggesting a larger role of sweat glands in clearing waste products or toxicants from the body (e.g. concentrations in sweat greater than that of blood) may be an artifact of methodological issues rather than evidence for selective transport.
The utility of sweat composition as a biomarker for human physiology is currently limited; more research is needed to determine potential relations between sweat and blood solute concentrations.
Biography
Lindsay B. Baker, PhD, is Senior Principal Scientist at the Gatorade Sports Science Institute (GSSI) and a PepsiCo R&D Associate Fellow. Lindsay has been conducting sports nutrition, hydration, and sweat studies for the GSSI research program since 2007. She is a Fellow of the American College of Sports Medicine and is on the Scientific Advisory Board for the Korey Stringer Institute.

Funding Statement
This work was supported by the Gatorade Sports Science Institute, a division of PepsiCo, Inc.
Abbreviations
ASGD activated sweat gland density AVP arginine vasopressin ATP adenosine triphosphate BPA bisphenol-A Ca calcium CFTR cystic fibrosis transmembrane conductance regulator Cl chloride Cu copper Ereq evaporative requirement for heat balance Fe iron HCO3 bicarbonate K potassium Mg magnesium Mn mangenese Na sodium NH3 ammonia RSR regional sweating rate SGO sweat gland output WBSR whole body sweating rate Zn zinc
Disclosure statement
The author is employed by the Gatorade Sports Science Institute, a division of PepsiCo, Inc. The views expressed in this article are those of the authors and do not necessarily reflect the position or policy of PepsiCo, Inc.
References
[1] Casa DJ, Cheuvront SN, Galloway SD, et al. Fluid needs for training, competition, and recovery in track-and-field athletes . Int J Sport Nutr Exerc Metab . 2019; 29 ( 2 ):175–180. [PubMed] [Google Scholar]
[2] Shibasaki M, Davis SL.. Human perspiration and cutaneous circulation In: Meyer F, Szygula Z, Wilk B, editors. Fluid balance, hydration, and athletic performance . Boca Raton (FL): CRC Press; 2016. p. 33–58. [Google Scholar]
[3] Sato K, Kang WH, Saga K, et al. Biology of sweat glands and their disorders. II. Disorders of sweat gland function . J Am Acad Dermatol . 1989; 20 ( 5 Pt 1 ):713–726. [PubMed] [Google Scholar]
[4] McLellan TM, Daanen HAM, Cheung SS. Encapsulated environment . Compr Physiol . 2013; 3 ( 3 ):1363–1391. [PubMed] [Google Scholar]
[5] Sato K. The physiology and pharmacology of the eccrine sweat gland In: Goldsmith L, editor. Biochemistry and physiology of the skin . New York: Oxford University Press; 1983. p. 596–641. [Google Scholar]
[6] Sato K. The physiology, pharmacology, and biochemistry of the eccrine sweat gland . Rev Physiol Biochem Pharmacol . 1977; 79 :51–131. [PubMed] [Google Scholar]
[7] Costill DL. Sweating: its composition and effects on body fluids . Ann N Y Acad Sci . 1977; 301 :160–174. [PubMed] [Google Scholar]
[8] Sato K, Sato F. Na+, K+, H+, Cl-, and Ca2+ concentrations in cystic fibrosis eccrine sweat in vivo and in vitro . J Lab Clin Med . 1990; 115 ( 4 ):504–511. [PubMed] [Google Scholar]
[9] Sato K. The mechanism of eccrine sweat secretion In: Gisolfi DRLCV, Nadel ER, editors. Exercise, heat, and thermoregulation . Dubuque (IA): Brown & Benchmark; 1993. p. 85–117. [Google Scholar]
[10] Sato K, Ohtsuyama M, Samman G. Eccrine sweat gland disorders . J Am Acad Dermatol . 1991; 24 ( 6 Pt 1 ):1010–1014. [PubMed] [Google Scholar]
[11] Montagna W, Parakkal PF. Eccrine sweat glands In: Montagna W, Parakkal PF, editors. The structure and function of skin . New York (NY): Academic Press, Inc; 1974. p. 366–411. [Google Scholar]
[13] Kuno Y. Human perspiration . Springfield (IL): Charles C. Thomas Publisher; 1956. [Google Scholar]
[14] Kuno Y. Variations in secretory activity of human sweat glands . Lancet . 1938; 1 :299–303. [Google Scholar]
[15] Sato K, Kang WH, Saga K, et al. Biology of sweat glands and their disorders. I. Normal sweat gland function . J Am Acad Dermatol . 1989; 20 ( 4 ):537–563. [PubMed] [Google Scholar]
[16] Taylor NA, Machado-Moreira CA. Regional variations in transepidermal water loss, eccrine sweat gland density, sweat secretion rates and electrolyte composition in resting and exercising humans . Extrem Physiol Med . 2013; 2 ( 1 ):4. [PMC free article] [PubMed] [Google Scholar]
[17] Bar-Or O, Magnusson LI, Buskirk ER. Distribution of heat-activated sweat glands in obese and lean men and women . Hum Biol . 1968; 40 ( 2 ):235–248. [PubMed] [Google Scholar]
[18] Sato F, Owen M, Matthes R, et al. Functional and morphological changes in the eccrine sweat gland with heat acclimation . J Appl Physiol (1985) . 1990; 69 ( 1 ):232–236. [PubMed] [Google Scholar]
[19] Sato K, Dobson RL. Regional and individual variations in the function of the human eccrine sweat gland . J Invest Dermatol . 1970; 54 ( 6 ):443–449. [PubMed] [Google Scholar]
[20] Hibbs RG. Electron microscopy of human apocrine sweat glands . J Invest Dermatol . 1962; 38 :77–84. [PubMed] [Google Scholar]
[21] Montagna W, Parakkal PF. Apocrine glands In: Montagna W, Parakkal PF, editors. The structure and function of skin . New York (NY): Academic Press, Inc; 1974. p. 332–365. [Google Scholar]
[22] Robertshaw D. Apocrine sweat glands In: Goldsmith LA, editor. Biochemistry and physiology of the skin . New York: Oxford University Press, Inc; 1983. p. 642–653. [Google Scholar]
[23] Sato K, Leidal R, Sato F. Morphology and development of an apoeccrine sweat gland in human axillae . Am J Physiol . 1987; 252 ( 1 Pt 2 ):R166–80. [PubMed] [Google Scholar]
[24] Sato K, Sato F. Sweat secretion by human axillary apoeccrine sweat gland in vitro . Am J Physiol . 1987; 252 ( 1 Pt 2 ):R181–7. [PubMed] [Google Scholar]
[25] Hussain JN, Mantri N, Cohen MM. Working up a good sweat – the challenges of standardising sweat collection for metabolomics analysis . Clin Biochem Rev . 2017; 38 ( 1 ):13–34. [PMC free article] [PubMed] [Google Scholar]
[26] Montagna W, Parakkal PF. Sebaceous glands In: Montagna W, Parakkal PF, editors. The structure and function of skin . New York (NY): Academic Press, Inc; 1974. p. 280–331. [Google Scholar]
[27] Porter AM. Why do we have apocrine and sebaceous glands? J R Soc Med . 2001; 94 ( 5 ):236–237. [PMC free article] [PubMed] [Google Scholar]
[28] Strauss JS, Downing DT, Ebling FJ. Sebaceous glands In: Goldsmith LA, editor. Biochemistry and physiology of the skin . New York: Oxford University Press, Inc; 1983. p. 569–595. [Google Scholar]
[29] Lonsdale-Eccles A, Leonard N, Lawrence C. Axillary hyperhidrosis: eccrine or apocrine? Clin Exp Dermatol . 2003; 28 ( 1 ):2–7. [PubMed] [Google Scholar]
[30] Ebling FJ. Apocrine glands in health and disorder . Int J Dermatol . 1989; 28 ( 8 ):508–511. [PubMed] [Google Scholar]
[31] Inoue R, Sohara E, Rai T, et al. Immunolocalization and translocation of aquaporin-5 water channel in sweat glands . J Dermatol Sci . 2013; 70 ( 1 ):26–33. [PubMed] [Google Scholar]
[32] Nejsum LN, Kwon T-H, Jensen UB, et al. Functional requirement of aquaporin-5 in plasma membranes of sweat glands . Proc Natl Acad Sci U S A . 2002; 99 ( 1 ):511–516. [PMC free article] [PubMed] [Google Scholar]
[33] Xie L, Jin L, Feng J, et al. The expression of AQP5 and UTs in the sweat glands of uremic patients . Biomed Res Int . 2017; 2017 :8629783. [PMC free article] [PubMed] [Google Scholar]
[34] Quinton PM. Effects of some ion transport inhibitors on secretion and reabsorption in intact and perfused single human sweat glands . Pflugers Arch . 1981; 391 ( 4 ):309–313. [PubMed] [Google Scholar]
[35] Reddy MM, Quinton PM. Rapid regulation of electrolyte absorption in sweat duct . J Membr Biol . 1994; 140 ( 1 ):57–67. [PubMed] [Google Scholar]
[36] Choi JY, Muallem D, Kiselyov K, et al. Aberrant CFTR-dependent HCO3- transport in mutations associated with cystic fibrosis . Nature . 2001; 410 ( 6824 ):94–97. [PMC free article] [PubMed] [Google Scholar]
[37] Kaiser D, Songo-Williams R, Drack E. Hydrogen ion and electrolyte excretion of the single human sweat gland . Pflugers Arch . 1974; 349 ( 1 ):63–72. [PubMed] [Google Scholar]
[38] Sato K, Dobson RL. The effect of intracutaneous d-aldosterone and hydrocortisone on human eccrine sweat gland function . J Invest Dermatol . 1970; 54 ( 6 ):450–462. [PubMed] [Google Scholar]
[39] Buono MJ, Claros R, Deboer T, et al. Na+ secretion rate increases proportionally more than the Na+ reabsorption rate with increases in sweat rate . J Appl Physiol (1985) . 2008; 105 ( 4 ):1044–1048. [PubMed] [Google Scholar]
[40] Cage GW, Dobson RL. Sodium secretion and reabsorption in the human eccrine sweat gland . J Clin Invest . 1965; 44 :1270–1276. [PMC free article] [PubMed] [Google Scholar]
[41] Sato K, Dobson RL. Glucose metabolism of the isolated eccrine sweat gland. II. The relation between glucose metabolism and sodium transport . J Clin Invest . 1973; 52 ( 9 ):2166–2174. [PMC free article] [PubMed] [Google Scholar]
[42] Dobson RL, Sato K. The secretion of salt and water by the eccrine sweat gland . Arch Dermatol . 1972; 105 ( 3 ):366–370. [PubMed] [Google Scholar]
[43] Elizondo RS. Local control of eccrine sweat gland function . Fed Proc . 1973; 32 ( 5 ):1583–1587. [PubMed] [Google Scholar]
[44] Elizondo RS, Banerjee M, Bullard RW. Effect of local heating and arterial occlusion on sweat electrolyte content . J Appl Physiol . 1972; 32 ( 1 ):1–6. [PubMed] [Google Scholar]
[45] Weiner JS, Van Heyningen RE. Observations on lactate content of sweat . J Appl Physiol . 1952; 4 ( 9 ):734–744. [PubMed] [Google Scholar]
[46] Smiles KA, Elizondo RS, Barney CC. Sweating responses during changes of hypothalamic temperature in the rhesus monkey . J Appl Physiol . 1976; 40 ( 5 ):653–657. [PubMed] [Google Scholar]
[47] Wingo JE, Low DA, Keller DM, et al. Skin blood flow and local temperature independently modify sweat rate during passive heat stress in humans . J Appl Physiol . 2010; 109 ( 5 ):1301–1306. [PMC free article] [PubMed] [Google Scholar]
[48] Nadel ER, Bullard RW, Stolwijk JA. Importance of skin temperature in the regulation of sweating . J Appl Physiol . 1971; 31 ( 1 ):80–87. [PubMed] [Google Scholar]
[49] Nadel ER, Mitchell JW, Saltin B, et al. Peripheral modifications to the central drive for sweating . J Appl Physiol . 1971; 31 ( 6 ):828–833. [PubMed] [Google Scholar]
[50] Morris NB, Bain AR, Cramer MN, et al. Evidence that transient changes in sudomotor output with cold and warm fluid ingestion are independently modulated by abdominal, but not oral thermoreceptors . J Appl Physiol (1985) . 2014; 116 ( 8 ):1088–1095. [PMC free article] [PubMed] [Google Scholar]
[51] Morris NB, Jay O. Staying warm in the cold with a hot drink: the role of visceral thermoreceptors . Temperature . 2017; 4 ( 2 ):123–125. doi: 10.1080/23328940.2017.1299667 [PMC free article] [PubMed] [CrossRef] [Google Scholar]
[52] Todd G, Gordon CJ, Groeller H, et al. Does intramuscular thermal feedback modulate eccrine sweating in exercising humans? Acta Physiol (Oxf) . 2014; 212 ( 1 ):86–96. [PubMed] [Google Scholar]
[53] Sato K. Stimulation of pentose cycle in the eccrine sweat gland by adrenergic drugs . Am J Physiol . 1973; 224 ( 5 ):1149–1154. [PubMed] [Google Scholar]
[54] Sawka MN, Leon LR, Montain SJ, et al. Integrated physiological mechanisms of exercise performance, adaptation, and maladaptation to heat stress . Compr Physiol . 2011; 1 ( 4 ):1883–1928. [PubMed] [Google Scholar]
[55] Shibasaki M, Crandall CG. Mechanisms and controllers of eccrine sweating in humans . Front Biosci (Schol Ed) . 2010; 2 :685–696. [PMC free article] [PubMed] [Google Scholar]
[56] Shibasaki M, Kondo N, Crandall CG. Non-thermoregulatory modulation of sweating in humans . Exerc Sport Sci Rev . 2003; 31 ( 1 ):34–39. [PubMed] [Google Scholar]
[57] Kondo N, Shibasaki M, Aoki K, et al. Function of human eccrine sweat glands during dynamic exercise and passive heat stress . J Appl Physiol (1985) . 2001; 90 ( 5 ):1877–1881. [PubMed] [Google Scholar]
[58] Kondo N, Takano S, Aoki K, et al. Regional differences in the effect of exercise intensity on thermoregulatory sweating and cutaneous vasodilation . Acta Physiol Scand . 1998; 164 ( 1 ):71–78. [PubMed] [Google Scholar]
[59] Randall WC. Quantitation and Regional Distribution of Sweat Glands in Man . J Clin Invest . 1946; 25 ( 5 ):761–767. [PMC free article] [PubMed] [Google Scholar]
[60] Armstrong LE, Maresh CM. Effects of training, environment, and host factors on the sweating response to exercise . Int J Sports Med . 1998; 19 Suppl 2 :S103–5. [PubMed] [Google Scholar]
[61] Nadel ER. Control of sweating rate while exercising in the heat . Med Sci Sports . 1979; 11 ( 1 ):31–35. [PubMed] [Google Scholar]
[62] Kirby CR, Convertino VA. Plasma aldosterone and sweat sodium concentrations after exercise and heat acclimation . J Appl Physiol . 1986; 61 ( 3 ):967–970. [PubMed] [Google Scholar]
[63] Allan JR, Wilson CG. Influence of acclimatization on sweat sodium concentration . J Appl Physiol . 1971; 30 ( 5 ):708–712. [PubMed] [Google Scholar]
[64] Pandolf KB, Cadarette BS, Sawka MN, et al. Thermoregulatory responses of middle-aged and young men during dry-heat acclimation . J Appl Physiol (1985) . 1988; 65 ( 1 ):65–71. [PubMed] [Google Scholar]
[65] Wenger CB. Human heat acclimitization . Pandolf KB, Sawka MN, Gonzalez RR, Editors. Indianapolis: Benchmark Press; 1988. p. 153–197. [Google Scholar]
[66] Greenleaf JE, Castle BL, Ruff WK. Maximal oxygen uptake, sweating and tolerance to exercise in the heat . Int J Biometeorol . 1972; 16 ( 4 ):375–387. [PubMed] [Google Scholar]
[67] Inoue Y, Havenith G, Kenney WL, et al. Exercise- and methylcholine-induced sweating responses in older and younger men: effect of heat acclimation and aerobic fitness . Int J Biometeorol . 1999; 42 ( 4 ):210–216. [PubMed] [Google Scholar]
[68] Buono MJ, McKenzie BK, Kasch FW. Effects of ageing and physical training on the peripheral sweat production of the human eccrine sweat gland . Age Ageing . 1991; 20 ( 6 ):439–441. [PubMed] [Google Scholar]
[69] Buono MJ, Sjoholm NT. Effect of physical training on peripheral sweat production . J Appl Physiol (1985) . 1988; 65 ( 2 ):811–814. [PubMed] [Google Scholar]
[70] Taylor NA. Human heat adaptation . Compr Physiol . 2014; 4 ( 1 ):325–365. [PubMed] [Google Scholar]
[71] Roberts MF, Wenger CB, Stolwijk JA, et al. Skin blood flow and sweating changes following exercise training and heat acclimation . J Appl Physiol Respir Environ Exerc Physiol . 1977; 43 ( 1 ):133–137. [PubMed] [Google Scholar]
[72] Nadel ER, Pandolf KB, Roberts MF, et al. Mechanisms of thermal acclimation to exercise and heat . J Appl Physiol . 1974; 37 ( 4 ):515–520. [PubMed] [Google Scholar]
[73] Lee J-B, Kim T-W, Min Y-K, et al. Long distance runners present upregulated sweating responses than sedentary counterparts . PLoS One . 2014; 9 ( 4 ):e93976. [PMC free article] [PubMed] [Google Scholar]
[74] Baum E, Bruck K, Schwennicke HP. Adaptive modifications in the thermoregulatory system of long-distance runners . J Appl Physiol . 1976; 40 ( 3 ):404–410. [PubMed] [Google Scholar]
[75] Buono MJ, White CS, Connolly KP. Cholinergic sensitivity of the eccrine sweat gland in trained and untrained men . J Dermatol Sci . 1992; 4 ( 1 ):33–37. [PubMed] [Google Scholar]
[76] Sawka MN, Young AJ, Francesconi RP, et al. Thermoregulatory and blood responses during exercise at graded hypohydration levels . J Appl Physiol . 1985; 59 ( 5 ):1394–1401. [PubMed] [Google Scholar]
[77] Montain SJ, Latzka WA, Sawka MN. Control of thermoregulatory sweating is altered by hydration level and exercise intensity . J Appl Physiol . 1995; 79 ( 5 ):1434–1439. [PubMed] [Google Scholar]
[78] Fortney SM, Wenger CB, Bove JR, et al. Effect of hyperosmolality on control of blood flow and sweating . J Appl Physiol . 1984; 57 ( 6 ):1688–1695. [PubMed] [Google Scholar]
[79] Libert JP, Candas V, Amoros C, et al. Local sweating responses of different body areas in dehydration-hydration experiments . J Physiol (Paris) . 1988; 83 ( 1 ):19–25. [PubMed] [Google Scholar]
[80] Lynn AG, Gagnon D, Binder K, et al. Divergent roles of plasma osmolality and the baroreflex on sweating and skin blood flow . Am J Physiol Regul Integr Comp Physiol . 2012; 302 ( 5 ):R634–42. [PubMed] [Google Scholar]
[81] Ito T, Itoh T, Hayano T, et al. Plasma hyperosmolality augments peripheral vascular response to baroreceptor unloading during heat stress . Am J Physiol Regul Integr Comp Physiol . 2005; 289 ( 2 ):R432–R440. [PubMed] [Google Scholar]
[82] Fortney SM, Nadel ER, Wenger CB, et al. Effect of blood volume on sweating rate and body fluids in exercising humans . J Appl Physiol . 1981; 51 ( 6 ):1594–1600. [PubMed] [Google Scholar]
[83] Gagnon D, Crandall CG. Sweating as a heat loss thermoeffector . Handb Clin Neurol . 2018; 156 :211–232. [PubMed] [Google Scholar]
[84] Ellis FP, Exton-Smith AN, Foster KG, et al. Eccrine sweating and mortality during heat waves in very young and very old persons . Isr J Med Sci . 1976; 12 ( 8 ):815–817. [PubMed] [Google Scholar]
[85] Kenney WL, Fowler SR. Methylcholine-activated eccrine sweat gland density and output as a function of age . J Appl Physiol (1985) . 1988; 65 ( 3 ):1082–1086. [PubMed] [Google Scholar]
[86] Inoue Y. Longitudinal effects of age on heat-activated sweat gland density and output in healthy active older men . Eur J Appl Physiol Occup Physiol . 1996; 74 ( 1–2 ):72–77. [PubMed] [Google Scholar]
[87] Larose J, Boulay P, Sigal RJ, et al. Age-related decrements in heat dissipation during physical activity occur as early as the age of 40 . PLoS One . 2013; 8 ( 12 ):e83148. [PMC free article] [PubMed] [Google Scholar]
[88] Foster KG, Ellis FP, Doré C, et al. Sweat responses in the aged . Age Ageing . 1976; 5 ( 2 ):91–101. [PubMed] [Google Scholar]
[89] Inoue Y, Nakao M, Araki T, et al. Regional differences in the sweating responses of older and younger men . J Appl Physiol (1985) . 1991; 71 ( 6 ):2453–2459. [PubMed] [Google Scholar]
[90] Inoue Y, Shibasaki M. Regional differences in age-related decrements of the cutaneous vascular and sweating responses to passive heating . Eur J Appl Physiol Occup Physiol . 1996; 74 ( 1–2 ):78–84. [PubMed] [Google Scholar]
[91] Smith CJ, Alexander LM, Kenney WL. Nonuniform, age-related decrements in regional sweating and skin blood flow . Am J Physiol Regul Integr Comp Physiol . 2013; 305 ( 8 ):R877–85. [PMC free article] [PubMed] [Google Scholar]
[92] Inoue Y, Shibasaki M, Ueda H, et al. Mechanisms underlying the age-related decrement in the human sweating response . Eur J Appl Physiol Occup Physiol . 1999; 79 ( 2 ):121–126. [PubMed] [Google Scholar]
[93] Kenney WL, Munce TA. Invited review: aging and human temperature regulation . J Appl Physiol (1985) . 2003; 95 ( 6 ):2598–2603. [PubMed] [Google Scholar]
[94] Inbar O, Morris N, Epstein Y, et al. Comparison of thermoregulatory responses to exercise in dry heat among prepubertal boys, young adults and older males . Exp Physiol . 2004; 89 ( 6 ):691–700. [PubMed] [Google Scholar]
[95] Tankersley CG, Smolander J, Kenney WL, et al. Sweating and skin blood flow during exercise: effects of age and maximal oxygen uptake . J Appl Physiol (1985) . 1991; 71 ( 1 ):236–242. [PubMed] [Google Scholar]
[96] Best S, Caillaud C, Thompson M. The effect of ageing and fitness on thermoregulatory response to high-intensity exercise . Scand J Med Sci Sports . 2012; 22 ( 4 ):e29–37. [PubMed] [Google Scholar]
[97] Kenney WL. Thermoregulation at rest and during exercise in healthy older adults In: Holloszy JO, editor. Exercise and sport sciences reviews . Baltimore (MD): Williams & Wilkins; 1997. p. 41–76. [PubMed] [Google Scholar]
[98] Kenney WL, Anderson RK. Responses of older and younger women to exercise in dry and humid heat without fluid replacement . Med Sci Sports Exerc . 1988; 20 ( 2 ):155–160. [PubMed] [Google Scholar]
[99] Inoue Y, Ichinose-Kuwahara T, Funaki C, et al. Sex differences in acetylcholine-induced sweating responses due to physical training . J Physiol Anthropol . 2014; 33 :13. [PMC free article] [PubMed] [Google Scholar]
[100] Gagnon D, Crandall CG, Kenny GP. Sex differences in postsynaptic sweating and cutaneous vasodilation . J Appl Physiol (1985) . 2013; 114 ( 3 ):394–401. [PMC free article] [PubMed] [Google Scholar]
[101] Gagnon D, Kenny GP. Sex differences in thermoeffector responses during exercise at fixed requirements for heat loss . J Appl Physiol (1985) . 2012; 113 ( 5 ):746–757. [PubMed] [Google Scholar]
[102] Gagnon D, Kenny GP. Sex modulates whole-body sudomotor thermosensitivity during exercise . J Physiol . 2011; 589 ( Pt 24 ):6205–6217. [PMC free article] [PubMed] [Google Scholar]
[103] Bar-Or O. Thermoregulation in females from a life span perspective In: Bar-Or O, Lamb DR, Clarkson PM, editors. Exercise and the Female. A Life Span Approach . Carmel (IN): Cooper Publishing Group; 1996. p. 249–288. [Google Scholar]
[104] Smith CJ, Havenith G. Body mapping of sweating patterns in athletes: a sex comparison . Med Sci Sports Exerc . 2012; 44 ( 12 ):2350–2361. [PubMed] [Google Scholar]
[105] Sawka MN, Sawka MN, Burke LM, et al. American college of sports medicine position stand. Exercise and fluid replacement . Med Sci Sports Exerc . 2007; 39 ( 2 ):377–390. [PubMed] [Google Scholar]
[106] Avellini BA, Shapiro Y, Pandolf KB, et al. Physiological responses of men and women to prolonged dry heat exposure . Aviat Space Environ Med . 1980; 51 ( 10 ):1081–1085. [PubMed] [Google Scholar]
[107] Shapiro Y, Pandolf KB, Avellini BA, et al. Physiological responses of men and women to humid and dry heat . J Appl Physiol . 1980; 49 ( 1 ):1–8. [PubMed] [Google Scholar]
[108] Havenith G, van Middendorp H. The relative influence of physical fitness, acclimatization state, anthropometric measures and gender on individual reactions to heat stress . Eur J Appl Physiol Occup Physiol . 1990; 61 ( 5–6 ):419–427. [PubMed] [Google Scholar]
[109] Notley SR, Park J, Tagami K, et al. Variations in body morphology explain sex differences in thermoeffector function during compensable heat stress . Exp Physiol . 2017; 102 ( 5 ):545–562. [PubMed] [Google Scholar]
[110] Charkoudian N, Stachenfeld N. Sex hormone effects on autonomic mechanisms of thermoregulation in humans . Auton Neurosci . 2016; 196 :75–80. [PubMed] [Google Scholar]
[111] Iyoho AE, Ng LJ, MacFadden L. Modeling of gender differences in thermoregulation . Mil Med . 2017; 182 ( S1 ):295–303. [PubMed] [Google Scholar]
[112] Kenney WL. A review of comparative responses of men and women to heat stress . Environ Res . 1985; 37 ( 1 ):1–11. [PubMed] [Google Scholar]
[113] Charkoudian N, Stachenfeld NS. Reproductive hormone influences on thermoregulation in women . Compr Physiol . 2014; 4 ( 2 ):793–804. [PubMed] [Google Scholar]
[114] Gagnon D, Kenny GP. Does sex have an independent effect on thermoeffector responses during exercise in the heat? J Physiol . 2012; 590 ( 23 ):5963–5973. [PMC free article] [PubMed] [Google Scholar]
[115] Rowland T. Thermoregulation during exercise in the heat in children: old concepts revisited . J Appl Physiol . 2008; 105 ( 2 ):718–724. [PubMed] [Google Scholar]
[116] Meyer F, Bar-Or O, MacDougall D, et al. Sweat electrolyte loss during exercise in the heat: effects of gender and maturation . Med Sci Sports Exerc . 1992; 24 ( 7 ):776–781. [PubMed] [Google Scholar]
[117] Falk B, Bar-Or O, Calvert R, et al. Sweat gland response to exercise in the heat among pre-, mid-, and late-pubertal boys . Med Sci Sports Exerc . 1992; 24 ( 3 ):313–319. [PubMed] [Google Scholar]
[118] Kolka MA, Stephenson LA, Rock PB, et al. Local sweating and cutaneous blood flow during exercise in hypobaric environments . J Appl Physiol (1985) . 1987; 62 ( 6 ):2224–2229. [PubMed] [Google Scholar]
[119] DiPasquale DM, Kolkhorst FW, Nichols JF, et al. Effect of acute normobaric hypoxia on peripheral sweat rate . High Alt Med Biol . 2002; 3 ( 3 ):289–292. [PubMed] [Google Scholar]
[120] Kacin A, Golja P, Eiken O, et al. The influence of acute and 23 days of intermittent hypoxic exposures on the exercise-induced forehead sweating response . Eur J Appl Physiol . 2007; 99 ( 5 ):557–566. [PubMed] [Google Scholar]
[121] Wenger CB, Roberts MF, Stolwijk JA, et al. Nocturnal lowering of thresholds for sweating and vasodilation . J Appl Physiol . 1976; 41 ( 1 ):15–19. [PubMed] [Google Scholar]
[122] Stephenson LA, Kolka MA. Menstrual cycle phase and time of day alter reference signal controlling arm blood flow and sweating . Am J Physiol . 1985; 249 ( 2 Pt 2 ):R186–91. [PubMed] [Google Scholar]
[123] Inoue Y, Tanaka Y, Omori K, et al. Sex- and menstrual cycle-related differences in sweating and cutaneous blood flow in response to passive heat exposure . Eur J Appl Physiol . 2005; 94 ( 3 ):323–332. [PubMed] [Google Scholar]
[124] Kuwahara T, Inoue Y, Taniguchi M, et al. Effects of physical training on heat loss responses of young women to passive heating in relation to menstrual cycle . Eur J Appl Physiol . 2005; 94 ( 4 ):376–385. [PubMed] [Google Scholar]
[125] Kolka MA, Stephenson LA. Control of sweating during the human menstrual cycle . Eur J Appl Physiol Occup Physiol . 1989; 58 ( 8 ):890–895. [PubMed] [Google Scholar]
[126] Horvath SM, Drinkwater BL. Thermoregulation and the menstrual cycle . Aviat Space Environ Med . 1982; 53 ( 8 ):790–794. [PubMed] [Google Scholar]
[127] Sunderland C, Nevill M. Effect of the menstrual cycle on performance of intermittent, high-intensity shuttle running in a hot environment . Eur J Appl Physiol . 2003; 88 ( 4–5 ):345–352. [PubMed] [Google Scholar]
[128] Janse XAK, Thompson MW, Chuter VH, et al. Exercise performance over the menstrual cycle in temperate and hot, humid conditions . Med Sci Sports Exerc . 2012; 44 ( 11 ):2190–2198. [PubMed] [Google Scholar]
[129] Notley SR, Dervis S, Poirier MP, et al. Menstrual cycle phase does not modulate whole body heat loss during exercise in hot, dry conditions . J Appl Physiol (1985) . 2019; 126 ( 2 ):286–293. [PMC free article] [PubMed] [Google Scholar]
[130] Lei TH, Mundel T. Humid heat stress affects trained female athletes more than does their menstrual phase . Temperature . 2018; 5 ( 3 ):202–204. doi: 10.1080/23328940.2018.1436394 [PMC free article] [PubMed] [CrossRef] [Google Scholar]
[131] Sato K, Sato F. Individual variations in structure and function of human eccrine sweat gland . Am J Physiol . 1983; 245 ( 2 ):R203–8. [PubMed] [Google Scholar]
[132] Baker LB. Sweating rate and sweat sodium concentration in athletes: A review of methodology and intra/interindividual variability . Sports Med . 2017; 47 ( Suppl 1 ):111–128. [PMC free article] [PubMed] [Google Scholar]
[133] Arn KD, Reimer A. Minimal sodium losses through the skin . J Clin Invest . 1950; 29 ( 10 ):1342–1346. [PMC free article] [PubMed] [Google Scholar]
[134] Robinson S, Robinson AH. Chemical composition of sweat . Physiol Rev . 1954; 34 ( 2 ):202–220. [PubMed] [Google Scholar]
[135] Freyberg RH, Grant RL. Loss of minerals through the skin of normal humans when sweating is avoided . J Clin Invest . 1937; 16 ( 5 ):729–731. [PMC free article] [PubMed] [Google Scholar]
[136] Souza SL, Graca G, Oliva A. Characterization of sweat induced with pilocarpine, physical exercise, and collected passively by metabolomic analysis . Skin Res Technol . 2018; 24 ( 2 ):187–195. [PubMed] [Google Scholar]
[137] Delgado-Povedano MM, Calderón-Santiago M, Luque de Castro MD, et al. Metabolomics analysis of human sweat collected after moderate exercise . Talanta . 2018; 177 :47–65. [PubMed] [Google Scholar]
[138] Harshman SW, Pitsch RL, Smith ZK, et al. The proteomic and metabolomic characterization of exercise-induced sweat for human performance monitoring: A pilot investigation . PLoS One . 2018; 13 ( 11 ):e0203133. [PMC free article] [PubMed] [Google Scholar]
[139] Stefaniak AB, Harvey CJ. Dissolution of materials in artificial skin surface film liquids . Toxicol In Vitro . 2006; 20 ( 8 ):1265–1283. [PubMed] [Google Scholar]
[140] Gordon RS Jr., Thompson RH, Muenzer J, et al. Sweat lactate in man is derived from blood glucose . J Appl Physiol . 1971; 31 ( 5 ):713–716. [PubMed] [Google Scholar]
[141] Steckl AJ, Ray P. Stress biomarkers in biological fluids and their point-of-use detection . ACS Sens . 2018; 3 ( 10 ):2025–2044. [PubMed] [Google Scholar]
[142] Jia M, Chew WM, Feinstein Y, et al. Quantification of cortisol in human eccrine sweat by liquid chromatography – tandem mass spectrometry . Analyst . 2016; 141 ( 6 ):2053–2060. [PMC free article] [PubMed] [Google Scholar]
[143] Harvey CJ, LeBouf RF, Stefaniak AB. Formulation and stability of a novel artificial human sweat under conditions of storage and use . Toxicol In Vitro . 2010; 24 ( 6 ):1790–1796. [PubMed] [Google Scholar]
[144] Shirreffs SM, Maughan RJ. Whole body sweat collection in humans: an improved method with preliminary data on electrolyte content . J Appl Physiol . 1997; 82 ( 1 ):336–341. [PubMed] [Google Scholar]
[145] Maughan R.J. and Shirreffs S.M.. Fluid and electrolyte loss and replacement in exercise In: Harries M, Williams C, Stanish WD, Micheli L, editors. Oxford textbook of sports medicine . New York (USA): Oxford University Press; 1998. p. 97–113. [Google Scholar]
[146] Baker LB, Stofan JR, Hamilton AA, et al. Comparison of regional patch collection vs. whole body washdown for measuring sweat sodium and potassium loss during exercise . J Appl Physiol . 2009; 107 ( 3 ):887–895. [PubMed] [Google Scholar]
[147] Patterson MJ, Galloway SD, Nimmo MA. Variations in regional sweat composition in normal human males . Exp Physiol . 2000; 85 ( 6 ):869–875. [PubMed] [Google Scholar]
[148] Verde T, Shephard RJ, Corey P, et al. Sweat composition in exercise and in heat . J Appl Physiol . 1982; 53 ( 6 ):1540–1545. [PubMed] [Google Scholar]
[149] Baker LB, Ungaro CT, Sopeña BC, et al. Body map of regional versus whole body sweating rate and sweat electrolyte concentrations in men and women during moderate exercise-heat stress . J Appl Physiol . 2018;124:1304–1318. [PubMed] [Google Scholar]
[150] Yoshida T, Shin-ya H, Nakai S, et al. Genomic and non-genomic effects of aldosterone on the individual variation of the sweat Na+ concentration during exercise in trained athletes . Eur J Appl Physiol . 2006; 98 ( 5 ):466–471. [PubMed] [Google Scholar]
[151] Brown MB, Haack KKV, Pollack BP, et al. Low abundance of sweat duct Cl- channel CFTR in both healthy and cystic fibrosis athletes with exceptionally salty sweat during exercise . Am J Physiol Regul Integr Comp Physiol . 2011; 300 ( 3 ):R605–15. [PMC free article] [PubMed] [Google Scholar]
[152] Johnson RE, Pitts GC, Consolazio FC. Factors influencing chloride concentration in human sweat . Am J Physiol . 1944; 141 :575–589. [Google Scholar]
[153] Lichton IJ. Osmotic pressure of human sweat . J Appl Physiol . 1957; 11 ( 3 ):422–424. [PubMed] [Google Scholar]
[154] Dill DB, Hall FG, Edwards HT. Changes in composition of sweat during acclimatization to heat . Am J Physiol . 1938; 123 :412–419. [Google Scholar]
[155] Shamsuddin AK, Yanagimoto S, Kuwahara T, et al. Changes in the index of sweat ion concentration with increasing sweat during passive heat stress in humans . Eur J Appl Physiol . 2005; 94 ( 3 ):292–297. [PubMed] [Google Scholar]
[156] Baker LB, Barnes KA, Anderson ML, et al. Normative data for regional sweat sodium concentration and whole-body sweating rate in athletes . J Sports Sci . 2016; 34 ( 4 ):358–368. [PubMed] [Google Scholar]
[157] Sawka MN. Physiological consequences of hypohydration: exercise performance and thermoregulation . Med Sci Sports Exerc . 1992; 24 ( 6 ):657–670. [PubMed] [Google Scholar]
[158] Robinson S, Gerking SD, Turrell ES, et al. Effect of skin temperature on salt concentration of sweat . J Appl Physiol . 1950; 2 ( 12 ):654–662. [PubMed] [Google Scholar]
[159] Baker LB, De Chavez PJD, Ungaro CT, et al. Exercise intensity effects on total sweat electrolyte losses and regional vs. whole-body sweat [Na(+)], [Cl(-)], and [K(+)] . Eur J Appl Physiol . 201. 9;119(2):361–375. [PMC free article] [PubMed] [Google Scholar]
[160] Collins KJ, Crockford GW, Weiner JS. The local training effect of secretory activity on the response of eccrine sweat glands . J Physiol . 1966; 184 ( 1 ):203–214. [PMC free article] [PubMed] [Google Scholar]
[161] Derbyshire PJ, Barr H, Davis F, et al. Lactate in human sweat: a critical review of research to the present day . J Physiol Sci . 2012; 62 ( 6 ):429–440. [PubMed] [Google Scholar]
[162] Buono MJ, Lee NV, Miller PW. The relationship between exercise intensity and the sweat lactate excretion rate . J Physiol Sci . 2010; 60 ( 2 ):103–107. [PubMed] [Google Scholar]
[163] Falk B, Bar-Or O, MacDougall JD, et al. Sweat lactate in exercising children and adolescents of varying physical maturity . J Appl Physiol (1985) . 1991; 71 ( 5 ):1735–1740. [PubMed] [Google Scholar]
[164] Gahm N, Shwachman H. Studies in cystic fibrosis of the pancreas; a simple test for the detection of excessive chloride on the skin . N Engl J Med . 1956; 255 ( 21 ):999–1001. [PubMed] [Google Scholar]
[165] Di Sant’Agnese PA, Darling RC, Perera GA, et al. Abnormal electrolyte composition of sweat in cystic fibrosis of the pancreas; clinical significance and relationship to the disease . Pediatrics . 1953; 12 ( 5 ):549–563. [PubMed] [Google Scholar]
[166] Gibson LE, Cooke RE. A test for concentration of electrolytes in sweat in cystic fibrosis of the pancreas utilizing pilocarpine by iontophoresis . Pediatrics . 1959; 23 ( 3 ):545–549. [PubMed] [Google Scholar]
[167] Rowe SM, Miller S, Sorscher EJ. Cystic fibrosis . N Engl J Med . 2005; 352 ( 19 ):1992–2001. [PubMed] [Google Scholar]
[168] Goodman BE, Percy WH. CFTR in cystic fibrosis and cholera: from membrane transport to clinical practice . Adv Physiol Educ . 2005; 29 ( 2 ):75–82. [PubMed] [Google Scholar]
[169] Eichner ER. Genetic and other determinants of sweat sodium . Curr Sports Med Rep . 2008; 7 :S36–S40. [Google Scholar]
[170] Reddy MM, Quinton PM. Functional interaction of CFTR and ENaC in sweat glands . Pflugers Arch . 2003; 445 ( 4 ):499–503. [PubMed] [Google Scholar]
[171] Beauchamp M, Lands LC. Sweat-testing: a review of current technical requirements . Pediatr Pulmonol . 2005; 39 ( 6 ):507–511. [PubMed] [Google Scholar]
[172] Mishra A, Greaves R, Smith K, et al. Diagnosis of cystic fibrosis by sweat testing: age-specific reference intervals . J Pediatr . 2008; 153 ( 6 ):758–763. [PubMed] [Google Scholar]
[173] Traeger N, Shi Q, Dozor AJ. Relationship between sweat chloride, sodium, and age in clinically obtained samples . J Cyst Fibros . 2014; 13 ( 1 ):10–14. [PubMed] [Google Scholar]
[174] Davé S, Honney S, Raymond J, et al. An unusual presentation of cystic fibrosis in an adult . Am J Kidney Dis . 2005; 45 ( 3 ):e41–4. [PubMed] [Google Scholar]
[175] Priou-Guesdon M, Malinge M-C, Augusto J-F, et al. Hypochloremia and hyponatremia as the initial presentation of cystic fibrosis in three adults . Ann Endocrinol (Paris) . 2010; 71 ( 1 ):46–50. [PubMed] [Google Scholar]
[176] Smith HR, Dhatt GS, Melia WM, et al. Cystic fibrosis presenting as hyponatraemic heat exhaustion . BMJ . 1995; 310 ( 6979 ):579–580. [PMC free article] [PubMed] [Google Scholar]
[177] Farrell PM, Koscik RE. Sweat chloride concentrations in infants homozygous or heterozygous for F508 cystic fibrosis . Pediatrics . 1996; 97 ( 4 ):524–528. [PubMed] [Google Scholar]
[178] Collie JTB, Massie RJ, Jones OAH, et al. Sixty-five years since the New York heat wave: advances in sweat testing for cystic fibrosis . Pediatr Pulmonol . 2014; 49 ( 2 ):106–117. [PubMed] [Google Scholar]
[179] Quinton PM. Physiological basis of cystic fibrosis: a historical perspective . Physiol Rev . 1999; 79 ( 1 Suppl ):S3–S22. [PubMed] [Google Scholar]
[180] Quinton PM. Cystic fibrosis: lessons from the sweat gland . Physiology (Bethesda) . 2007; 22 :212–225. [PubMed] [Google Scholar]
[181] Gao W, Brooks GA, Klonoff DC. Wearable physiological systems and technologies for metabolic monitoring . J Appl Physiol (1985) . 2018; 124 ( 3 ):548–556. [PubMed] [Google Scholar]
[182] Heikenfeld J, Jajack A, Rogers J, et al. Wearable sensors: modalities, challenges, and prospects . Lab Chip . 2018; 18 ( 2 ):217–248. [PMC free article] [PubMed] [Google Scholar]
[183] Boysen TC, Yanagawa S, Sato F, et al. A modified anaerobic method of sweat collection . J Appl Physiol . 1984; 56 ( 5 ):1302–1307. [PubMed] [Google Scholar]
[184] Prasad AS, Schulert AR, Sandstead HH, et al. Zinc, iron, and nitrogen content of sweat in normal and deficient subjects . J Lab Clin Med . 1963; 62 :84–89. [PubMed] [Google Scholar]
[185] Hussain R, Patwardhan VN. Iron content of thermal sweat in iron-deficiency anaemia . Lancet . 1959; 1 ( 7082 ):1073–1074. [PubMed] [Google Scholar]
[186] Zhou Y, Han H, Naw HPP, et al. Real-time colorimetric hydration sensor for sport activities . Mater Des . 2016; 90 :1181–1185. [Google Scholar]
[187] Gao W, Emaminejad S, Nyein HYY, et al. Fully integrated wearable sensor arrays for multiplexed in situ perspiration analysis . Nature . 2016; 529 ( 7587 ):509–514. [PMC free article] [PubMed] [Google Scholar]
[188] Alizadeh A, Burns A, Lenigk R, et al. A wearable patch for continuous monitoring of sweat electrolytes during exertion . Lab Chip . 2018; 18 ( 17 ):2632–2641. [PubMed] [Google Scholar]
[189] Rose DP, Ratterman ME, Griffin DK, et al. Adhesive RFID sensor patch for monitoring of sweat electrolytes . IEEE Trans Biomed Eng . 2015; 62 ( 6 ):1457–1465. [PubMed] [Google Scholar]
[190] Morgan RM, Patterson MJ, Nimmo MA. Acute effects of dehydration on sweat composition in men during prolonged exercise in the heat . Acta Physiol Scand . 2004; 182 ( 1 ):37–43. [PubMed] [Google Scholar]
[191] Costill DL, Cote R, Fink W. Muscle water and electrolytes following varied levels of dehydration in man . J Appl Physiol . 1976; 40 ( 1 ):6–11. [PubMed] [Google Scholar]
[192] Armstrong LE, Hubbard RW, Szlyk PC, et al. Voluntary dehydration and electrolyte losses during prolonged exercise in the heat . Aviat Space Environ Med . 1985; 56 ( 8 ):765–770. [PubMed] [Google Scholar]
[193] Amatruda TT, Welt LG. Secretion of electrolytes in thermal sweat . J Appl Physiol . 1953; 5 :759–772. [Google Scholar]
[194] Walsh RM, Noakes TD, Hawley JA, et al. Impaired high-intensity cycling performance time at low levels of dehydration . Int J Sports Med . 1994; 15 ( 7 ):392–398. [PubMed] [Google Scholar]
[195] Robinson S, Maletich RT, Robinson WS, et al. Output of NaCl by sweat glands and kidneys in relation to dehydration and to salt depletion . J Appl Physiol . 1956; 8 ( 6 ):615–620. [PubMed] [Google Scholar]
[196] Joyner MJ, Coyle EF. Endurance exercise performance: the physiology of champions . J Physiol . 2008; 586 ( 1 ):35–44. [PMC free article] [PubMed] [Google Scholar]
[197] Wenger CB. Heat of evaporation of sweat: thermodynamic considerations . J Appl Physiol . 1972; 32 ( 4 ):456–459. [PubMed] [Google Scholar]
[198] Shapiro Y, Pandolf KB, Goldman RF. Predicting sweat loss response to exercise, environment and clothing . Eur J Appl Physiol Occup Physiol . 1982; 48 ( 1 ):83–96. [PubMed] [Google Scholar]
[199] Bain AR, Deren TM, Jay O. Describing individual variation in local sweating during exercise in a temperate environment . Eur J Appl Physiol . 2011; 111 ( 8 ):1599–1607. [PubMed] [Google Scholar]
[200] Gagge AP, Gonzalez RR. Mechanisms of heat exchange: biophysics and physiology In: Fregley MJ, Blatteis CM, editors. Handbook of physiology . New York (NY): Oxford University Press; 1996. p. 45–84. [Google Scholar]
[201] Gagnon D, Jay O, Kenny GP. The evaporative requirement for heat balance determines whole-body sweat rate during exercise under conditions permitting full evaporation . J Physiol . 2013; 591 ( Pt 11 ):2925–2935. [PMC free article] [PubMed] [Google Scholar]
[202] Nielsen M. Die Regulation der Korpertemperatur beiMuskelarbeit . Skand Arch Physiol . 1938; 79 :193–230. [Google Scholar]
[203] Cramer MN, Jay O. Explained variance in the thermoregulatory responses to exercise: the independent roles of biophysical and fitness/fatness-related factors . J Appl Physiol (1985) . 2015;119:982–989. [PMC free article] [PubMed] [Google Scholar]
[204] Sawka MN, Castellani JW, Cheuvront SN, et al. Physiologic systems and their responses to conditions of heat and cold In: Farrell PA, Joyner MJ, Caiozzo VJeditors. ACSM’s advanced exercise physiology . Baltimore (MD): Lippincott Williams & Wilkins; 2012. p. 567–602. [Google Scholar]
[205] Sawka MN, Wenger CB. Physiological responses to acute exercise-heat stress In: Pandolf KB, Sawka MN, Gonzalez RR, editors. Human performance physiology and environmental medicine at terrestrial extremes . Carmel (IN): Cooper Publishing Group; 1988. p. 97–151. [Google Scholar]
[206] Watabe A, Sugawara T, Kikuchi K, et al. Sweat constitutes several natural moisturizing factors, lactate, urea, sodium, and potassium . J Dermatol Sci . 2013; 72 ( 2 ):177–182. [PubMed] [Google Scholar]
[207] Dunstan RH, Sparkes DL, Dascombe BJ, et al. Sweat facilitated amino acid losses in male athletes during exercise at 32-34 degrees C . PLoS One . 2016; 11 ( 12 ):e0167844. [PMC free article] [PubMed] [Google Scholar]
[208] Gerrett N, Griggs K, Redortier B, et al. Sweat from gland to skin surface: production, transport, and skin absorption . J Appl Physiol (1985) . 2018; 125 ( 2 ):459–469. [PubMed] [Google Scholar]
[209] Shiohara T, Mizukawa Y, Shimoda-Komatsu Y, et al. Sweat is a most efficient natural moisturizer providing protective immunity at points of allergen entry . Allergol Int . 2018; 67 ( 4 ):442–447. [PubMed] [Google Scholar]
[210] Lobitz WC Jr., Mason HL. Chemistry of palmar sweat; discussion of studies on chloride, urea, glucose, uric acid, ammonia nitrogen and creatinine . Arch Derm Syphilol . 1948; 57 ( 5 ):907–915. [PubMed] [Google Scholar]
[211] Schittek B, Hipfel R, Sauer B, et al. Dermcidin: a novel human antibiotic peptide secreted by sweat glands . Nat Immunol . 2001; 2 ( 12 ):1133–1137. [PubMed] [Google Scholar]
[212] Murakami M, Ohtake T, Dorschner RA, et al. Cathelicidin anti-microbial peptide expression in sweat, an innate defense system for the skin . J Invest Dermatol . 2002; 119 ( 5 ):1090–1095. [PubMed] [Google Scholar]
[213] Park J-H, Park G-T, Cho IH, et al. An antimicrobial protein, lactoferrin exists in the sweat: proteomic analysis of sweat . Exp Dermatol . 2011; 20 ( 4 ):369–371. [PubMed] [Google Scholar]
[214] Murota H, Matsui S, Ono E, et al. Sweat, the driving force behind normal skin: an emerging perspective on functional biology and regulatory mechanisms . J Dermatol Sci . 2015; 77 ( 1 ):3–10. [PubMed] [Google Scholar]
[215] Shiohara T, Sato Y, Komatsu Y, et al. Sweat as an efficient natural moisturizer . Curr Probl Dermatol . 2016; 51 :30–41. [PubMed] [Google Scholar]
[216] Schröder JM, Harder J. Antimicrobial skin peptides and proteins . Cell Mol Life Sci . 2006; 63 ( 4 ):469–486. [PubMed] [Google Scholar]
[217] Buono MJ, Kolding M, Leslie E, et al. Heat acclimation causes a linear decrease in sweat sodium ion concentration . J Therm Biol . 2018; 71 :237–240. [PubMed] [Google Scholar]
[218] Buono MJ, Ball KD, Kolkhorst FW. Sodium ion concentration vs. sweat rate relationship in humans . J Appl Physiol . 2007; 103 ( 3 ):990–994. [PubMed] [Google Scholar]
[219] Chinevere TD, Kenefick RW, Cheuvront SN, et al. Effect of heat acclimation on sweat minerals . Med Sci Sports Exerc . 2008; 40 ( 5 ):886–891. [PubMed] [Google Scholar]
[220] Nielsen B, Strange S, Christensen NJ, et al. Acute and adaptive responses in humans to exercise in a warm, humid environment . Pflugers Arch . 1997; 434 ( 1 ):49–56. [PubMed] [Google Scholar]
[221] Smith CJ, Havenith G. Upper body sweat mapping provides evidence of relative sweat redistribution towards the periphery following hot-dry heat acclimation . Temperature . 2019; 6 :50–65. doi: 10.1080/23328940.2019.1570777 [PMC free article] [PubMed] [CrossRef] [Google Scholar]
[222] Poirier MP, Gagnon D, Kenny GP. Local versus whole-body sweating adaptations following 14 days of traditional heat acclimation . Appl Physiol Nutr Metab . 2016; 41 ( 8 ):816–824. [PubMed] [Google Scholar]
[223] Ladell WSS, Shephard RJ. Aldosterone inhibition and acclimatization to heat . Proc Physiol Soc . 1961; 3–4 :19P–20P. [Google Scholar]
[224] Armstrong LE, Costill DL, Fink WJ, et al. Effects of dietary sodium on body and muscle potassium content during heat acclimation . Eur J Appl Physiol Occup Physiol . 1985; 54 ( 4 ):391–397. [PubMed] [Google Scholar]
[225] McCance RA. The effect of salt deficiency in man on the volume of the extracellular fluids, and on the composition of sweat, saliva, gastric juice and cerebrospinal fluid . J Physiol . 1938; 92 ( 2 ):208–218. [PMC free article] [PubMed] [Google Scholar]
[226] Klesges RC, Ward KD, Shelton ML, et al. Changes in bone mineral content in male athletes. Mechanisms of action and intervention effects . JAMA . 1996; 276 ( 3 ):226–230. [PubMed] [Google Scholar]
[227] Ely MR, Kenefick RW, Cheuvront SN, et al. The effect of heat acclimation on sweat microminerals: artifact of surface contamination . Int J Sport Nutr Exerc Metab . 2013; 23 ( 5 ):470–479. [PubMed] [Google Scholar]
[228] Ely MR, Kenefick RW, Cheuvront SN, et al. Surface contamination artificially elevates initial sweat mineral concentrations . J Appl Physiol . 2011; 110 ( 6 ):1534–1540. [PubMed] [Google Scholar]
[229] Montain SJ, Cheuvront SN, Lukaski HC. Sweat mineral-element responses during 7 h of exercise-heat stress . Int J Sport Nutr Exerc Metab . 2007; 17 ( 6 ):574–582. [PubMed] [Google Scholar]
[230] DeRuisseau KC, Cheuvront SN, Haymes EM, et al. Sweat iron and zinc losses during prolonged exercise . Int J Sport Nutr Exerc Metab . 2002; 12 ( 4 ):428–437. [PubMed] [Google Scholar]
[231] Mitchell HH, Hamilton TS. The dermal excretion under controlled environmental conditions of nitrogen and minerals in human subjects, with particular reference to calcium and iron . J Biol Chem . 1949; 178 ( 1 ):345–361. [PubMed] [Google Scholar]
[232] Waller MF, Haymes EM. The effects of heat and exercise on sweat iron loss . Med Sci Sports Exerc . 1996; 28 ( 2 ):197–203. [PubMed] [Google Scholar]
[233] Paulev PE, Jordal R, Pedersen NS. Dermal excretion of iron in intensely training athletes . Clin Chim Acta . 1983; 127 ( 1 ):19–27. [PubMed] [Google Scholar]
[234] Brune M, Magnusson B, Persson H, et al. Iron losses in sweat . Am J Clin Nutr . 1986; 43 ( 3 ):438–443. [PubMed] [Google Scholar]
[235] Costa F, Calloway DH, Margen S. Regional and total body sweat composition of men fed controlled diets . Am J Clin Nutr . 1969; 22 ( 1 ):52–58. [PubMed] [Google Scholar]
[236] Hargreaves M, Morgan TO, Snow R, et al. Exercise tolerance in the heat on low and normal salt intakes . Clin Sci (Lond) . 1989; 76 ( 5 ):553–557. [PubMed] [Google Scholar]
[237] McCubbin AJ, Costa RJS. The impact of dietary sodium intake on sweat sodium concenctration in response to endurance exercise: a systematic review . Int J Sports Sci . 2018; 8 :25–37. [Google Scholar]
[238] Robinson S, Nicholas JR, Smith JH, et al. Time relation of renal and sweat gland adjustments to salt deficiency in men . J Appl Physiol . 1955; 8 ( 2 ):159–165. [PubMed] [Google Scholar]
[239] Komives GK, Robinson S, Roberts JT. Urea transfer across the sweat glands . J Appl Physiol . 1966; 21 ( 6 ):1681–1684. [PubMed] [Google Scholar]
[240] Sigal CB, Dobson RL. The effect of salt intake on sweat gland function . J Invest Dermatol . 1968; 50 ( 6 ):451–455. [PubMed] [Google Scholar]
[241] Allsopp AJ, Sutherland R, Wood P, et al. The effect of sodium balance on sweat sodium secretion and plasma aldosterone concentration . Eur J Appl Physiol Occup Physiol . 1998; 78 ( 6 ):516–521. [PubMed] [Google Scholar]
[242] Konikoff F, Shoenfeld Y, Magazanik A, et al. Effects of salt loading during exercise in a hot dry climate . Biomed Pharmacother . 1986; 40 ( 8 ):296–300. [PubMed] [Google Scholar]
[243] Koenders EE, Franken CPG, Cotter JD, et al. Restricting dietary sodium reduces plasma sodium response to exercise in the heat . Scand J Med Sci Sports . 2017; 27 ( 11 ):1213–1220. [PubMed] [Google Scholar]
[244] Costill DL, Coté R, Miller E, et al. Water and electrolyte replacement during repeated days of work in the heat . Aviat Space Environ Med . 1975; 46 ( 6 ):795–800. [PubMed] [Google Scholar]
[245] Hamouti N, Fernández-Elías VE, Ortega JF, et al. Ingestion of sodium plus water improves cardiovascular function and performance during dehydrating cycling in the heat . Scand J Med Sci Sports . 2012;24:507–518. [PubMed] [Google Scholar]
[246] USDA What we eat in America, NHANES 2013-2014 . [cited 2019 March24]. Available from: https://www.ars.usda.gov/ARSUserFiles/80400530/pdf/1314/Table_1_NIN_GEN_13.pdf.
[247] McCubbin AJ, Costa RJS. The impact of dietary sodium intake on sweat sodium concentration in response to endurance exercise: a systematic review . Int J Sports Sci . 2018; 8 ( 1 ):25–37. [Google Scholar]
[248] Jacob RA, Sandstead HH, Munoz JM, et al. Whole body surface loss of trace metals in normal males . Am J Clin Nutr . 1981; 34 ( 7 ):1379–1383. [PubMed] [Google Scholar]
[249] Wheeler EF, el-Neil H, Willson JO, et al. The effect of work level and dietary intake on water balance and the excretion of sodium, potassium and iron in a hot climate . Br J Nutr . 1973; 30 ( 1 ):127–137. [PubMed] [Google Scholar]
[250] Lugg JW, Ellis FP. Some water-soluble vitamins in the sweat of tropically acclimatized European men . Br J Nutr . 1954; 8 ( 1 ):71–77. [PubMed] [Google Scholar]
[251] Vellar OD. Studies on sweat losses of nutrients. II. The influence of an oral iron load on the iron content of whole body cell-free sweat . Scand J Clin Lab Invest . 1968; 21 ( 4 ):344–346. [PubMed] [Google Scholar]
[252] Milne DB, Canfield WK, Mahalko JR, et al. Effect of dietary zinc on whole body surface loss of zinc: impact on estimation of zinc retention by balance method . Am J Clin Nutr . 1983; 38 ( 2 ):181–186. [PubMed] [Google Scholar]
[253] Green R, Charlton R, Seftel H, et al. Body iron excretion in man: a collaborative study . Am J Med . 1968; 45 ( 3 ):336–353. [PubMed] [Google Scholar]
[254] Weintraub LR, Demis DJ, Conrad ME, et al. Iron excretion by the skin. Selective localization of iron-59 in epithelial cells . Am J Pathol . 1965; 46 :121–127. [PMC free article] [PubMed] [Google Scholar]
[255] Nguyen MK, Kurtz I. New insights into the pathophysiology of the dysnatremias: a quantitative analysis . Am J Physiol Renal Physiol . 2004; 287 ( 2 ):F172–80. [PubMed] [Google Scholar]
[256] Hew-Butler T, Rosner MH, Fowkes-Godek S, et al. Statement of the 3rd international exercise-associated hyponatremia consensus development conference, Carlsbad, California, 2015 . Br J Sports Med . 2015; 49 ( 22 ):1432–1446. [PubMed] [Google Scholar]
[257] Montain SJ, Sawka MN, Wenger CB. Hyponatremia associated with exercise: risk factors and pathogenesis . Exerc Sport Sci Rev . 2001; 29 ( 3 ):113–117. [PubMed] [Google Scholar]
[258] Moss KN. Some effects of high air temperatures and muscular exertion upon colliers . Proc Roy Soc London B . 1923; 95 :181–200. [Google Scholar]
[259] Bates CM, Baum M, Quigley R. Cystic fibrosis presenting with hypokalemia and metabolic alkalosis in a previously healthy adolescent . J Am Soc Nephrol . 1997; 8 ( 2 ):352–355. [PubMed] [Google Scholar]
[260] O’Brien KK, Montain SJ, Corr WP, et al. Hyponatremia associated with overhydration in U.S. Army trainees . Mil Med . 2001; 166 ( 5 ):405–410. [PubMed] [Google Scholar]
[261] Institute of Medicine Water In: Dietary reference intakes for water, potassium, sodium, chloride, and sulfate . Washington (DC): National Academies Press; 2005. p. 73–185. [Google Scholar]
[262] Montain SJ, Cheuvront SN, Sawka MN. Exercise associated hyponatraemia: quantitative analysis to understand the aetiology . Br J Sports Med . 2006; 40 ( 2 ): 98–105. discussion 98-105. [PMC free article] [PubMed] [Google Scholar]
[263] Lewis D, Blow A, Tye J, et al. Considering exercise-associated hyponatraemia as a continuum . BMJ Case Rep 2018 . 2018. [PMC free article] [PubMed] [Google Scholar]
[264] Hiller WD. Dehydration and hyponatremia during triathlons . Med Sci Sports Exerc . 1989; 21 ( 5 Suppl ):S219–21. [PubMed] [Google Scholar]
[265] Scurati-Manzoni E, Fossali EF, Agostoni C, et al. Electrolyte abnormalities in cystic fibrosis: systematic review of the literature . Pediatr Nephrol . 2014; 29 ( 6 ):1015–1023. [PubMed] [Google Scholar]
[266] Leoni GB, Pitzalis S, Podda R, et al. A specific cystic fibrosis mutation (T3381) associated with the phenotype of isolated hypotonic dehydration . J Pediatr . 1995; 127 ( 2 ):281–283. [PubMed] [Google Scholar]
[267] Epaud R, Girodon E, Corvol H, et al. Mild cystic fibrosis revealed by persistent hyponatremia during the French 2003 heat wave, associated with the S1455X C-terminus CFTR mutation . Clin Genet . 2005; 68 ( 6 ):552–553. [PubMed] [Google Scholar]
[268] Godek SF, Peduzzi C, Burkholder R, et al. Sweat rates, sweat sodium concentrations, and sodium losses in 3 groups of professional football players . J Athl Train . 2010; 45 ( 4 ):364–371. [PMC free article] [PubMed] [Google Scholar]
[269] Horswill CA, Stofan JR, Lacambra M, et al. Sodium balance during U. S. football training in the heat: cramp-prone vs. reference players . Int J Sports Med . 2009; 30 ( 11 ):789–794. [PubMed] [Google Scholar]
[270] Augusto J-F, Sayegh J, Malinge M-C, et al. Severe episodes of extra cellular dehydration: an atypical adult presentation of cystic fibrosis . Clin Nephrol . 2008; 69 ( 4 ):302–305. [PubMed] [Google Scholar]
[271] Maughan RJ, Burke LM, Dvorak J, et al. IOC consensus statement: dietary supplements and the high-performance athlete . Br J Sports Med . 2018; 52 ( 7 ):439–455. [PMC free article] [PubMed] [Google Scholar]
[272] Beatty T, Webner D, Collina SJ. Bone density in competitive cyclists . Curr Sports Med Rep . 2010; 9 ( 6 ):352–355. [PubMed] [Google Scholar]
[273] Barry DW, Kohrt WM. BMD decreases over the course of a year in competitive male cyclists . J Bone Miner Res . 2008; 23 ( 4 ):484–491. [PubMed] [Google Scholar]
[274] Aruoma OI, Reilly T, MacLaren D, et al. Iron, copper and zinc concentrations in human sweat and plasma; the effect of exercise . Clin Chim Acta . 1988; 177 ( 1 ):81–87. [PubMed] [Google Scholar]
[275] Harrison ME, Walls C, Korslund MK, et al. An estimation of mineral losses through arm sweat of preadolescent children . Am J Clin Nutr . 1976; 29 ( 8 ):842–846. [PubMed] [Google Scholar]
[276] Vellar OD, Askevold R. Studies on sweat losses of nutrients. 3. Calcium, magnesium, and chloride content of whole body cell-free sweat in healthy unacclimatized men under controlled environmental conditions . Scand J Clin Lab Invest . 1968; 22 ( 1 ):65–71. [PubMed] [Google Scholar]
[277] O’Toole ML, Johnson KC, Satterfield S, et al. Do sweat calcium losses affect bone mass during firefighting training? J Occup Environ Med . 2000; 42 :1054–1059. [PubMed] [Google Scholar]
[278] Kohrt WM, Wherry SJ, Wolfe P, et al. Maintenance of serum ionized calcium during exercise attenuates parathyroid hormone and bone resorption responses . J Bone Miner Res . 2018; 33 ( 7 ):1326–1334. [PMC free article] [PubMed] [Google Scholar]
[279] Martin BR, Davis S, Campbell WW, et al. Exercise and calcium supplementation: effects on calcium homeostasis in sportswomen . Med Sci Sports Exerc . 2007; 39 ( 9 ):1481–1486. [PubMed] [Google Scholar]
[280] Hohnadel DC, Sunderman FW, Nechay MW, et al. Atomic absorption spectrometry of nickel, copper, zinc, and lead in sweat collected from healthy subjects during sauna bathing . Clin Chem . 1973; 19 ( 11 ):1288–1292. [PubMed] [Google Scholar]
[281] Lamanca JJ, Haymes EM, Daly JA, et al. Sweat iron loss of male and female runners during exercise . Int J Sports Med . 1988; 9 ( 1 ):52–55. [PubMed] [Google Scholar]
[282] Consolazio CF, Matoush LO, Nelson RA, et al. Relationship between calcium in sweat, calcium balance, and calcium requirements . J Nutr . 1962; 78 :78–88. [PubMed] [Google Scholar]
[283] Barry DW, Kohrt WM. Acute effects of 2 hours of moderate-intensity cycling on serum parathyroid hormone and calcium . Calcif Tissue Int . 2007; 80 :359–365. [PubMed] [Google Scholar]
[284] Tang Y-M, Wang D-G, Li J, et al. Relationships between micronutrient losses in sweat and blood pressure among heat-exposed steelworkers . Ind Health . 2016; 54 ( 3 ):215–223. [PMC free article] [PubMed] [Google Scholar]
[285] Cohn JR, Emmett EA. The excretion of trace metals in human sweat . Ann Clin Lab Sci . 1978; 8 ( 4 ):270–275. [PubMed] [Google Scholar]
[286] Baker LB, Stofan JR, Lukaski HC, et al. Exercise-induced trace mineral element concentration in regional versus whole-body wash-down sweat . Int J Sport Nutr Exerc Metab . 2011; 21 ( 3 ):233–239. [PubMed] [Google Scholar]
[287] Haakonssen EC, Ross ML, Knight EJ, et al. The effects of a calcium-rich pre-exercise meal on biomarkers of calcium homeostasis in competitive female cyclists: a randomised crossover trial . PLoS One . 2015; 10 ( 5 ):e0123302. [PMC free article] [PubMed] [Google Scholar]
[288] Barry DW, Hansen KC, van Pelt RE, et al. Acute calcium ingestion attenuates exercise-induced disruption of calcium homeostasis . Med Sci Sports Exerc . 2011; 43 ( 4 ):617–623. [PMC free article] [PubMed] [Google Scholar]
[289] Barry DW, Kohrt WM. Acute effects of 2 hours of moderate-intensity cycling on serum parathyroid hormone and calcium . Calcif Tissue Int . 2007; 80 ( 6 ):359–365. [PubMed] [Google Scholar]
[290] Labib M, Obeid D. Hyperhidrosis and iron deficiency . Ann Clin Biochem . 1995; 32 ( Pt 5 ):509–510. [PubMed] [Google Scholar]
[291] Nickerson HJ, Holubets MC, Weiler BR, et al. Causes of iron deficiency in adolescent athletes . J Pediatr . 1989; 114 ( 4 Pt 1 ):657–663. [PubMed] [Google Scholar]
[292] Clarkson PM, Haymes EM. Exercise and mineral status of athletes: calcium, magnesium, phosphorus, and iron . Med Sci Sports Exerc . 1995; 27 ( 6 ):831–843. [PubMed] [Google Scholar]
[293] Agu KA. Can sweat glands act as temporary or permanent replacement for the excretory function of the kidney? Emer Lif Sci Res . 2017; 3 ( 2 ):37–41. [Google Scholar]
[294] Rowell LB. Neural-humoral adjustments to orthostasis and long-term control In: Rowell LB, editor. Human Cardiovascular Control . New York: Oxford University Press; 1993. p. 81–117. [Google Scholar]
[295] Hew-Butler T, Noakes TD, Soldin SJ, et al. Acute changes in arginine vasopressin, sweat, urine and serum sodium concentrations in exercising humans: does a coordinated homeostatic relationship exist? Br J Sports Med . 2010; 44 ( 10 ):710–715. [PMC free article] [PubMed] [Google Scholar]
[296] Fasciolo JC, Totel GL, Johnson RE. Antidiuretic hormone and human eccrine sweating . J Appl Physiol . 1969; 27 ( 3 ):303–307. [PubMed] [Google Scholar]
[297] Hankiss J. Effect of antidiuretic hormone on sweating as a proof of its extrarenal action . Am J Med Sci . 1959; 238 :452–455. [PubMed] [Google Scholar]
[298] Schlein EM, Spooner GR, Day C, et al. Extrarenal water loss and antidiuretic hormone . J Appl Physiol . 1971; 31 ( 4 ):569–572. [PubMed] [Google Scholar]
[299] Pearcy M, ROBINSON S, MILLER DI, et al. Effects of dehydration, salt depletion and pitressin on sweat rate and urine flow . J Appl Physiol . 1956; 8 ( 6 ):621–626. [PubMed] [Google Scholar]
[300] Senay LC Jr., Van Beaumont W. Antidiuretic hormone and evaporative weight loss during heat stress . Pflugers Arch . 1969; 312 ( 3 ):82–90. [PubMed] [Google Scholar]
[301] Ratner AC, Dobson RL. The effect of antidiuretic hormone on sweating . J Invest Dermatol . 1964; 43 :379–381. [PubMed] [Google Scholar]
[302] Gibinski K, Kozłowski S, Chwalbińska-Moneta J, et al. ADH and thermal sweating . Eur J Appl Physiol Occup Physiol . 1979; 42 ( 1 ):1–13. [PubMed] [Google Scholar]
[303] Taussig LM, Braunstein GD. Effects of vasopressin on sweat rate and composition in patients with diabetes insipidus and normal controls . J Invest Dermatol . 1973; 60 ( 4 ):197–202. [PubMed] [Google Scholar]
[304] Ladell WSS, Whitcher HW. The effect of pituitrin on sweating . J Physiol . 1960; 154 :44–45P. [Google Scholar]
[305] Allen JA, Roddie IC. The effect of antidiuretic hormone on human sweating . J Physiol . 1974; 236 ( 2 ):403–412. [PMC free article] [PubMed] [Google Scholar]
[306] Hew-Butler T, Hummel J, Rider BC, et al. Characterization of the effects of the vasopressin V2 receptor on sweating, fluid balance, and performance during exercise . Am J Physiol Regul Integr Comp Physiol . 2014; 307 ( 4 ):R366–75. [PMC free article] [PubMed] [Google Scholar]
[307] Dobson RL. The human eccrine sweat gland: structural and functional interrelationships . Arch Environ Health . 1965; 11 :423–429. [PubMed] [Google Scholar]
[308] Fujii N, McNeely BD, Nishiyasu T, et al. Intradermal administration of atrial natriuretic peptide has no effect on sweating and cutaneous vasodilator responses in young male adults . Temperature . 2017; 4 ( 4 ):406–413. doi: 10.1080/23328940.2017.1356433 [PMC free article] [PubMed] [CrossRef] [Google Scholar]
[309] Crinnion WJ. Sauna as a valuable clinical tool for cardiovascular, autoimmune, toxicant- induced and other chronic health problems . Altern Med Rev . 2011; 16 ( 3 ):215–225. [PubMed] [Google Scholar]
[310] Hussain J, Cohen M. Clinical effects of regular dry sauna bathing: a systematic review . Evid Based Complement Alternat Med . 2018; 2018 :1857413. [PMC free article] [PubMed] [Google Scholar]
[311] Genuis SJ, Beesoon S, Birkholz D, et al. Human excretion of bisphenol A: blood, urine, and sweat (BUS) study . J Environ Public Health . 2012; 2012 :185731. [PMC free article] [PubMed] [Google Scholar]
[312] Genuis SJ, Lane K, Birkholz D. Human elimination of organochlorine pesticides: blood, urine, and sweat study . Biomed Res Int . 2016; 2016 :1624643. [PMC free article] [PubMed] [Google Scholar]
[313] Genuis SK, Birkholz D, Genuis SJ. Human excretion of polybrominated diphenyl ether flame retardants: blood, urine, and sweat study . Biomed Res Int . 2017; 2017 :3676089. [PMC free article] [PubMed] [Google Scholar]
[314] Sheng J, Qiu W, Xu B, et al. Monitoring of heavy metal levels in the major rivers and in residents’ blood in Zhenjiang City, China, and assessment of heavy metal elimination via urine and sweat in humans . Environ Sci Pollut Res Int . 2016; 23 ( 11 ):11034–11045. [PubMed] [Google Scholar]
[315] Lee D-H, Jacobs DR, Park HY, et al. A role of low dose chemical mixtures in adipose tissue in carcinogenesis . Environ Int . 2017; 108 :170–175. [PubMed] [Google Scholar]
[316] Genuis SJ, Beesoon S, Birkholz D. Biomonitoring and elimination of perfluorinated compounds and polychlorinated biphenyls through perspiration: blood, urine, and sweat study . ISRN Toxicol . 2013; 2013 :483832. [PMC free article] [PubMed] [Google Scholar]
[317] Genuis SJ, Beesoon S, Lobo RA, et al. Human elimination of phthalate compounds: blood, urine, and sweat (BUS) study . ScientificWorldJournal . 2012; 2012 :615068. [PMC free article] [PubMed] [Google Scholar]
[318] Genuis SJ, Birkholz D, Rodushkin I, et al. Blood, urine, and sweat (BUS) study: monitoring and elimination of bioaccumulated toxic elements . Arch Environ Contam Toxicol . 2011; 61 ( 2 ):344–357. [PubMed] [Google Scholar]
[319] Takemura T, Wertz PW, Sato K. Free fatty acids and sterols in human eccrine sweat . Br J Dermatol . 1989; 120 ( 1 ):43–47. [PubMed] [Google Scholar]
[320] Omokhodion FO, Crockford GW. Lead in sweat and its relationship to salivary and urinary levels in normal healthy subjects . Sci Total Environ . 1991; 103 ( 2–3 ):113–122. [PubMed] [Google Scholar]
[321] Lilley SG, Florence TM, Stauber JL. The use of sweat to monitor lead absorption through the skin . Sci Total Environ . 1988; 76 ( 2–3 ):267–278. [PubMed] [Google Scholar]
[322] Yousuf AK, Misbahuddin M, Rahman MS. Secretion of arsenic, cholesterol, vitamin E, and zinc from the site of arsenical melanosis and leucomelanosis in skin . Clin Toxicol (Phila) . 2011; 49 ( 5 ):374–378. [PubMed] [Google Scholar]
[323] Porucznik CA, Cox KJ, Wilkins DG, et al. A preliminary study of biomonitoring for bisphenol-A in human sweat . J Anal Toxicol . 2015; 39 ( 7 ):562–566. [PMC free article] [PubMed] [Google Scholar]
[324] Völkel W, Colnot T, Csanády GA, et al. Metabolism and kinetics of bisphenol a in humans at low doses following oral administration . Chem Res Toxicol . 2002; 15 ( 10 ):1281–1287. [PubMed] [Google Scholar]
[325] Rabinowitz MB, Wetherill GW, Kopple JD. Kinetic analysis of lead metabolism in healthy humans . J Clin Invest . 1976; 58 ( 2 ):260–270. [PMC free article] [PubMed] [Google Scholar]
[326] Rea WJ, Pan Y, Johnson AR. Clearing of toxic volatile hydrocarbons from humans . Bol Asoc Med P R . 1991; 83 ( 7 ):321–324. [PubMed] [Google Scholar]
[327] Lennox RD, Cecchini-Sternquist M. Safety and tolerability of sauna detoxification for the protracted withdrawal symptoms of substance abuse . J Int Med Res . 2018; 46 ( 11 ):4480–4499. [PMC free article] [PubMed] [Google Scholar]
[328] Ross GH, Sternquist MC. Methamphetamine exposure and chronic illness in police officers: significant improvement with sauna-based detoxification therapy . Toxicol Ind Health . 2012; 28 ( 8 ):758–768. [PMC free article] [PubMed] [Google Scholar]
[329] Penning R, McKinney A, Bus LD, et al. Measurement of alcohol hangover severity: development of the alcohol hangover severity scale (AHSS) . Psychopharmacology (Berl) . 2013; 225 ( 4 ):803–810. [PubMed] [Google Scholar]
[330] Ylikahri R, Heikkonen E, Soukas A. The sauna and alcohol . Ann Clin Res . 1988; 20 ( 4 ):287–291. [PubMed] [Google Scholar]
[331] Desruelle AV, Boisvert P, Candas V. Alcohol and its variable effect on human thermoregulatory response to exercise in a warm environment . Eur J Appl Physiol Occup Physiol . 1996; 74 ( 6 ):572–574. [PubMed] [Google Scholar]
[332] Allison TG, Reger WE. Thermoregulatory, cardiovascular, and psychophysical response to alcohol in men in 40 degrees C water . J Appl Physiol (1985) . 1992; 72 ( 6 ):2099–2107. [PubMed] [Google Scholar]
[333] Yoda T, Crawshaw LI, Nakamura M, et al. Effects of alcohol on thermoregulation during mild heat exposure in humans . Alcohol . 2005; 36 ( 3 ):195–200. [PubMed] [Google Scholar]
[334] Buono MJ. Sweat ethanol concentrations are highly correlated with co-existing blood values in humans . Exp Physiol . 1999; 84 ( 2 ):401–404. [PubMed] [Google Scholar]
[335] Phillips M, McAloon MH. A sweat-patch test for alcohol consumption: evaluation in continuous and episodic drinkers . Alcohol Clin Exp Res . 1980; 4 ( 4 ):391–395. [PubMed] [Google Scholar]
[336] Cederbaum AI. Alcohol metabolism . Clin Liver Dis . 2012; 16 ( 4 ):667–685. [PMC free article] [PubMed] [Google Scholar]
[337] al-Tamer YY, Hadi EA, al B II. Sweat urea, uric acid and creatinine concentrations in uraemic patients . Urol Res . 1997; 25 ( 5 ):337–340. [PubMed] [Google Scholar]
[338] Gorski J, Lerczak K, Wojcieszak I. Urea excretion in sweat during short-term efforts of high intensity . Eur J Appl Physiol Occup Physiol . 1985; 54 ( 4 ):416–419. [PubMed] [Google Scholar]
[339] Huang C-T, Chen M-L, Huang -L-L, et al. Uric acid and urea in human sweat . Chin J Physiol . 2002; 45 ( 3 ):109–115. [PubMed] [Google Scholar]
[340] Kenny GP, Stapleton JM, Yardley JE, et al. Older adults with type 2 diabetes store more heat during exercise . Med Sci Sports Exerc . 2013; 45 ( 10 ):1906–1914. [PubMed] [Google Scholar]
[341] Stapleton JM, Yardley JE, Boulay P, et al. Whole-body heat loss during exercise in the heat is not impaired in type 1 diabetes . Med Sci Sports Exerc . 2013; 45 ( 9 ):1656–1664. [PubMed] [Google Scholar]
[342] Carter MR, McGinn R, Barrera-Ramirez J, et al. Impairments in local heat loss in type 1 diabetes during exercise in the heat . Med Sci Sports Exerc . 2014; 46 ( 12 ):2224–2233. [PubMed] [Google Scholar]
[343] Fealey RD, Low PA, Thomas JE. Thermoregulatory sweating abnormalities in diabetes mellitus . Mayo Clin Proc . 1989; 64 ( 6 ):617–628. [PubMed] [Google Scholar]
[344] Kenny GP, Sigal RJ, McGinn R. Body temperature regulation in diabetes . Temperature . 2016; 3 ( 1 ):119–145.doi: 10.1080/23328940.2015.1131506 PMCID: PMC4861190 [PMC free article] [PubMed] [CrossRef] [Google Scholar]
[345] Conn JW. Electrolyte composition of sweat; clinical implications as an index of adrenal cortical function . Arch Intern Med (Chic) . 1949; 83 ( 4 ):416–428. [PubMed] [Google Scholar]
[346] Cheshire WP, Freeman R. Disorders of sweating . Semin Neurol . 2003; 23 ( 4 ):399–406. [PubMed] [Google Scholar]
[347] Davis SL, Shibasaki M, Low DA, et al. Skin grafting impairs postsynaptic cutaneous vasodilator and sweating responses . J Burn Care Res . 2007; 28 ( 3 ):435–441. [PMC free article] [PubMed] [Google Scholar]
[348] Davis SL, Shibasaki M, Low DA, et al. Impaired cutaneous vasodilation and sweating in grafted skin during whole-body heating . J Burn Care Res . 2007; 28 ( 3 ):427–434. [PMC free article] [PubMed] [Google Scholar]
[349] Davis SL, Shibasaki M, Low DA, et al. Sustained impairments in cutaneous vasodilation and sweating in grafted skin following long-term recovery . J Burn Care Res . 2009; 30 ( 4 ):675–685. [PMC free article] [PubMed] [Google Scholar]
[350] Pandolf KB, Gange RW, Latzka WA, et al. Human thermoregulatory responses during heat exposure after artificially induced sunburn . Am J Physiol . 1992; 262 ( 4 Pt 2 ):R610–6. [PubMed] [Google Scholar]
[351] Pandolf KB, Griffin TB, Munro EH, et al. Persistence of impaired heat tolerance from artificially induced miliaria rubra . Am J Physiol . 1980; 239 ( 3 ):R226–32. [PubMed] [Google Scholar]
[352] Pandolf KB, Griffin TB, Munro EH, et al. Heat intolerance as a function of percent of body surface involved with miliaria rubra . Am J Physiol . 1980; 239 ( 3 ):R233–40. [PubMed] [Google Scholar]
[353] Murota H, Yamaga K, Ono E, et al. Sweat in the pathogenesis of atopic dermatitis . Allergol Int . 2018; 67 ( 4 ):455–459. [PubMed] [Google Scholar]
[354] Matsui S, Murota H, Takahashi A, et al. Dynamic analysis of histamine-mediated attenuation of acetylcholine-induced sweating via GSK3beta activation . J Invest Dermatol . 2014; 134 ( 2 ):326–334. [PubMed] [Google Scholar]
[355] Cheshire WP, Fealey RD. Drug-induced hyperhidrosis and hypohidrosis: incidence, prevention and management . Drug Saf . 2008; 31 ( 2 ):109–126. [PubMed] [Google Scholar]
[356] Nawrocki S, Cha J. The etiology, diagnosis and management of hyperhidrosis: a comprehensive review. part II. Therapeutic options . J Am Acad Dermatol . 2019. [Google Scholar]
[357] Schlereth T, Dieterich M, Birklein F. Hyperhidrosis–causes and treatment of enhanced sweating . Dtsch Arztebl Int . 2009; 106 ( 3 ):32–37. [PMC free article] [PubMed] [Google Scholar]
[358] Mold JW, Holtzclaw BJ, McCarthy L. Night sweats: a systematic review of the literature . J Am Board Fam Med . 2012; 25 ( 6 ):878–893. [PubMed] [Google Scholar]
[359] Walling HW. Clinical differentiation of primary from secondary hyperhidrosis . J Am Acad Dermatol . 2011; 64 ( 4 ):690–695. [PubMed] [Google Scholar]
[360] Takamata A, Mack GW, Gillen CM, et al. Osmoregulatory modulation of thermal sweating in humans: reflex effects of drinking . Am J Physiol . 1995; 268 ( 2 Pt 2 ):R414–22. [PubMed] [Google Scholar]
[361] Cage GW, Wolfe SM, Thompson RH, et al. Effects of water intake on composition of thermal sweat in normal human volunteers . J Appl Physiol . 1970; 29 ( 5 ):687–690. [PubMed] [Google Scholar]
[362] Smith CJ, Havenith G. Body mapping of sweating patterns in male athletes in mild exercise-induced hyperthermia . Eur J Appl Physiol . 2011; 111 ( 7 ):1391–1404. [PubMed] [Google Scholar]
[363] Gonzalez RR, Cheuvront SN, Ely BR, et al. Sweat rate prediction equations for outdoor exercise with transient solar radiation . J Appl Physiol . 2012; 112 ( 8 ):1300–1310. [PubMed] [Google Scholar]
[364] Nielsen B, Kassow K, Aschengreen FE. Heat balance during exercise in the sun . Eur J Appl Physiol Occup Physiol . 1988; 58 ( 1–2 ):189–196. [PubMed] [Google Scholar]
[365] Gagge AP, Hardy JD. Thermal radiation exchange of the human by partitional calorimetry . J Appl Physiol . 1967; 23 ( 2 ):248–258. [PubMed] [Google Scholar]
[366] Adams WC, Mack GW, Langhans GW, et al. Effects of varied air velocity on sweating and evaporative rates during exercise . J Appl Physiol (1985) . 1992; 73 ( 6 ):2668–2674. [PubMed] [Google Scholar]
[367] Shaffrath JD, Adams WC. Effects of airflow and work load on cardiovascular drift and skin blood flow . J Appl Physiol Respir Environ Exerc Physiol . 1984; 56 ( 5 ):1411–1417. [PubMed] [Google Scholar]
[368] Adams WC. Influence of exercise mode and selected ambient conditions on skin temperature . Ann N Y Acad Sci . 1977; 301 :110–127. [PubMed] [Google Scholar]
[369] Collins KJ, Weiner JS. Observations on arm-bag suppression of sweating and its relationship to thermal sweat-gland ‘fatigue’ . J Physiol . 1962; 161 :538–556. [PMC free article] [PubMed] [Google Scholar]
[370] Randall WC, Peiss CN. The relationship between skin hydration and the suppression of sweating . J Invest Dermatol . 1957; 28 ( 6 ):435–441. [PubMed] [Google Scholar]
[371] Candas V, Libert JP, Vogt JJ. Sweating and sweat decline of resting men in hot humid environments . Eur J Appl Physiol Occup Physiol . 1983; 50 ( 2 ):223–234. [PubMed] [Google Scholar]
[372] Dziedzic CE, Ross ML, Slater GJ, et al. Variability of measurements of sweat sodium using the regional absorbent patch method . Int J Sports Physiol Perform . 2014; 9 :832–838. [PubMed] [Google Scholar]
[373] Kolka MA, Stephenson LA, Gonzalez RR. Depressed sweating during exercise at altitude . J Therm Biol . 1989; 14 :167–170. [Google Scholar]
[374] Gonzalez RR, Kenefick RW, Muza SR, et al. Sweat rate and prediction validation during high-altitude treks on Mount Kilimanjaro . J Appl Physiol (1985) . 2013; 114 ( 4 ):436–443. [PubMed] [Google Scholar]
[375] Westerterp KR, Robach P, Wouters L, et al. Water balance and acute mountain sickness before and after arrival at high altitude of 4,350 m . J Appl Physiol (1985) . 1996; 80 ( 6 ):1968–1972. [PubMed] [Google Scholar]
[376] Varene P, Jacquemin C, Durand J, et al. Energy balance during moderate exercise at altitude . J Appl Physiol . 1973; 34 ( 5 ):633–638. [PubMed] [Google Scholar]
[377] Buskirk ER. Body fluid volumes in relation to altitude, exercise and cold exposure. Arctic Aeromed. Lab., Fort Wainwright, Alaska . Proc Symp on Arctic Med Biol . 1966;375–413. [Google Scholar]
[378] Houdas Y, LeCroart JL, Ledru C, et al. Thermal sweat rate response to an acute short exposure at a simulated altitude of 4,600m . Aviat Space Environ Med . 1979; 50 ( 1 ):60–62. [PubMed] [Google Scholar]
[379] Armstrong LE, Johnson EC, Casa DJ, et al. The American football uniform: uncompensable heat stress and hyperthermic exhaustion . J Athl Train . 2010; 45 ( 2 ):117–127. [PMC free article] [PubMed] [Google Scholar]
[380] Mathews DK, Fox EL, Tanzi D. Physiological responses during exercise and recovery in a football uniform . J Appl Physiol . 1969; 26 ( 5 ):611–615. [PubMed] [Google Scholar]
[381] Dennis SC, Noakes TD. Advantages of a smaller bodymass in humans when distance running in warm humid conditions . Eur J Appl Physiol . 1999; 79 :280–284. [PubMed] [Google Scholar]
[382] Marino FE, Mbambo Z, Kortekaas E, et al. Advantages of smaller body mass during distance running in warm, humid environments . Pflugers Arch . 2000; 441 ( 2–3 ):359–367. [PubMed] [Google Scholar]
[383] Buresh R, Berg K, Noble J. Heat production and storage are positively correlated with measures of body size/composition and heart rate drift during vigorous running . Res Q Exerc Sport . 2005; 76 ( 3 ):267–274. [PubMed] [Google Scholar]
[384] Deren TM, Coris EE, Bain AR, et al. Sweating is greater in NCAA football linemen independently of heat production . Med Sci Sports Exerc . 2012; 44 ( 2 ):244–252. [PubMed] [Google Scholar]
[385] Nielsen B, Hales JR, Strange S, et al. Human circulatory and thermoregulatory adaptations with heat acclimation and exercise in a hot, dry environment . J Physiol . 1993; 460 :467–485. [PMC free article] [PubMed] [Google Scholar]
[386] Morimoto T, Slabochova Z, Naman RK, et al. Sex differences in physiological reactions to thermal stress . J Appl Physiol . 1967; 22 ( 3 ):526–532. [PubMed] [Google Scholar]
[387] Green JM, Bishop PA, Muir IH, et al. Gender differences in sweat lactate . Eur J Appl Physiol . 2000; 82 ( 3 ):230–235. [PubMed] [Google Scholar]
[388] Brown G, Dobson RL. Sweat sodium excretion in normal women . J Appl Physiol . 1967; 23 ( 1 ):97–99. [PubMed] [Google Scholar]
[389] Wells CL, Horvath SM. Responses to exercise in a hot environment as related to the menstrual cycle . J Appl Physiol . 1974; 36 ( 3 ):299–302. [PubMed] [Google Scholar]
[390] McLean A, Brown RC, Black KE. The fluid and electrolyte balance of New Zealand European and Maori/Pacific Island athletes: an observational study . Eur J Sport Sci . 2016; 16 ( 3 ):336–343. [PubMed] [Google Scholar]
[391] Kawahata A, Sakamoto H. Some observations on sweating of the Aino . Jpn J Physiol . 1951; 2 ( 2 ):166–169. [PubMed] [Google Scholar]
[393] Bae J-S, Lee J-B, Matsumoto T, et al. Prolonged residence of temperate natives in the tropics produces a suppression of sweating . Pflugers Arch . 2006; 453 ( 1 ):67–72. [PubMed] [Google Scholar]
[394] Candas V. Adaptation to extreme environments. Thermophysiological changes in man during humid heat acclimation In: Dejours P, editor. Comparative physiology of environmental adaptations . Basel (Switzerland): Karger; 1987. p. 76–93. [Google Scholar]
[395] Lee J-B, Bae J-S, Matsumoto T, et al. Tropical Malaysians and temperate Koreans exhibit significant differences in sweating sensitivity in response to iontophoretically administered acetylcholine . Int J Biometeorol . 2009; 53 ( 2 ):149–157. [PubMed] [Google Scholar]
[396] Lee JB, Kim JH, Murota H. Perspiration functions in different ethnic, age, and sex populations: modification of sudomotor function . Curr Probl Dermatol . 2016; 51 :109–119. [PubMed] [Google Scholar]
[397] Lee J, Shin Y. Comparison of density and output of sweat gland in tropical Africans and temperate Koreans . Auton Neurosci . 2017; 205 :67–71. [PubMed] [Google Scholar]
[398] Russell E, Koren G, Rieder M, et al. The detection of cortisol in human sweat: implications for measurement of cortisol in hair . Ther Drug Monit . 2014; 36 ( 1 ):30–34. [PubMed] [Google Scholar]
[399] Sato K, Sato F. Interleukin-1 alpha in human sweat is functionally active and derived from the eccrine sweat gland . Am J Physiol . 1994; 266 ( 3 Pt 2 ):R950–9. [PubMed] [Google Scholar]
[400] Ament W, Huizenga JR, Mook GA, et al. Lactate and ammonia concentration in blood and sweat during incremental cycle ergometer exercise . Int J Sports Med . 1997; 18 ( 1 ):35–39. [PubMed] [Google Scholar]
[401] Nyman E, Palmlov A. The elimination of ethyl alcohol in sweat . Skand Arch Physiol . 1936; 74 :155–159. [Google Scholar]
[402] Ikai K, Sato K, Sugiyama K, et al. Comparison of human sweat electrolyte concentration in mental, thermal and exercise perspiration . Nagoya Med J . 1969; 15 ( 1 ):47–66. [PubMed] [Google Scholar]
[403] Haymes EM. Mineral sweat losses during exercise In: Institute of Medicine, editor. Mineral requirements for military personnel . Washington (DC): National Academies Press; 2006. p. 323–329. [Google Scholar]
[404] Collins KJ. Composition of palmar and forearm sweat . J Appl Physiol . 1962; 17 :99–102. [PubMed] [Google Scholar]
[405] Hjortskov N, Jepsen LT, Nielsen B, et al. Pilocarpine iontophoresis test: an index of physiological sweat secretion? Clin Physiol . 1995; 15 ( 4 ):409–414. [PubMed] [Google Scholar]
[406] Vimieiro-Gomes AC, Magalhães FC, Amorim FT, et al. Comparison of sweat rate during graded exercise and the local rate induced by pilocarpine . Braz J Med Biol Res . 2005; 38 ( 7 ):1133–1139. [PubMed] [Google Scholar]
[407] Sato K, Feibleman C, Dobson RL. The electrolyte composition of pharmacologically and thermally stimulated sweat: a comparative study . J Invest Dermatol . 1970; 55 ( 6 ):433–438. [PubMed] [Google Scholar]
[408] Schwachman H, Antonowicz I. The sweat test in cystic fibrosis . Ann New York Acad Sci . 1962; 93 :600–624. [Google Scholar]
[409] Lemon PW, Yarasheski KE. Feasibility of sweat collection by whole body washdown in moderate to high humidity environments . Int J Sports Med . 1985; 6 ( 1 ):41–43. [PubMed] [Google Scholar]
[410] Candas V, Libert JP, Vogt JJ. Human skin wettedness and evaporative efficiency of sweating . J Appl Physiol Respir Environ Exerc Physiol . 1979; 46 ( 3 ):522–528. [PubMed] [Google Scholar]
[411] Havenith G, Fogarty A, Bartlett R, et al. Male and female upper body sweat distribution during running measured with technical absorbents . Eur J Appl Physiol . 2008; 104 ( 2 ):245–255. [PubMed] [Google Scholar]
[412] Maughan RJ, Dargavel LA, Hares R, et al. Water and salt balance of well-trained swimmers in training . Int J Sport Nutr Exerc Metab . 2009; 19 ( 6 ):598–606. [PubMed] [Google Scholar]
[413] Brisson GR, Boisvert P, Péronnet F, et al. A simple and disposable sweat collector . Eur J Appl Physiol Occup Physiol . 1991; 63 ( 3–4 ):269–272. [PubMed] [Google Scholar]
[414] Ely MR, Ely BR, Chinevere TD, et al. Evaluation of the Megaduct sweat collector for mineral analysis . Physiol Meas . 2012; 33 ( 3 ):385–394. [PubMed] [Google Scholar]
[415] Brengelmann GL, McKeag M, Rowell LB. Use of dew-point detection for quantitative measurement of sweating rate . J Appl Physiol . 1975; 39 ( 3 ):498–500. [PubMed] [Google Scholar]
[416] Graichen H, Rascati R, Gonzalez RR. Automatic dew-point temperature sensor . J Appl Physiol Respir Environ Exerc Physiol . 1982; 52 ( 6 ):1658–1660. [PubMed] [Google Scholar]
[417] Boisvert P, Desruelle AV, Candas V. Comparison of sweat rate measured by a pouch collector and a hygrometric technique during exercise . Can J Appl Physiol . 1997; 22 ( 2 ):161–170. [PubMed] [Google Scholar]
[418] Morris NB, Cramer MN, Hodder SG, et al. A comparison between the technical absorbent and ventilated capsule methods for measuring local sweat rate . J Appl Physiol (1985) . 2013; 114 ( 6 ):816–823. [PubMed] [Google Scholar]
[419] Kenefick RW, Cheuvront SN, Elliott LD, et al. Biological and analytical variation of the human sweating response: implications for study design and analysis . Am J Physiol Regul Integr Comp Physiol . 2012; 302 ( 2 ):R252–8. [PubMed] [Google Scholar]
[420] Weiner JS, Van Heyningen R. Lactic acid and sweat gland function . Nature . 1949; 164 ( 4165 ):351. [PubMed] [Google Scholar]
[421] Van Heyningen R, Weiner JS. A comparison of arm-bag sweat and body sweat . J Physiol . 1952; 116 ( 4 ):395–403. [PMC free article] [PubMed] [Google Scholar]
[422] Omokhodion FO, Howard JM. Trace elements in the sweat of acclimatized persons . Clin Chim Acta . 1994; 231 ( 1 ):23–28. [PubMed] [Google Scholar]
[423] Yokozeki H, Sato K. Thiol-dependent aminopeptidase (350,000 mol wt) as a marker of epidermal contamination in sweat . J Appl Physiol (1985) . 1987; 63 ( 3 ):1040–1048. [PubMed] [Google Scholar]
[424] Bergeron J, Bachmann LM, Miller WG. Influence of sample storage conditions on sweat chloride results . Clin Chem . 2011; 57 ( 4 ):641–643. [PubMed] [Google Scholar]
[425] Baker LB, Barnes KA, Sopeña BC, et al. Sweat sodium, potassium, and chloride concentrations analyzed same day as collection versus after 7 days storage in a range of temperatures . Int J Sport Nutr Exerc Metab . 2018; 28 ( 3 ):238–245. [PubMed] [Google Scholar]
[426] Goulet ED, Asselin A, Gosselin J, et al. Measurement of sodium concentration in sweat samples: comparison of five analytical techniques . Appl Physiol Nutr Metab . 2017; 42 :861–868. [PubMed] [Google Scholar]
[427] Boisvert P, Candas V. Validity of the Wescor’s sweat conductivity analyzer for the assessment of sweat electrolyte concentrations . Eur J Appl Physiol Occup Physiol . 1994; 69 ( 2 ):176–178. [PubMed] [Google Scholar]
[428] Goulet ED, Dion T, Myette-Cote E. Validity and reliability of the Horiba C-122 compact sodium analyzer in sweat samples of athletes . Eur J Appl Physiol . 2012; 112 :3479–3485. [PubMed] [Google Scholar]
[429] Baker LB, Ungaro CT, Barnes KA, et al. Validity and reliability of a field technique for sweat Na+ and K+ analysis during exercise in a hot-humid environment . Physiol Rep . 2014; 2 ( 5 ):e12007. [PMC free article] [PubMed] [Google Scholar]
[430] Doorn J, Storteboom TTR, Mulder AM, et al. Ion chromatography for the precise analysis of chloride and sodium in sweat for the diagnosis of cystic fibrosis . Ann Clin Biochem . 2015; 52 ( Pt 4 ):421–427. [PubMed] [Google Scholar]
[431] Thienpont LM, Van Nuwenborg JE, Reinauer H, et al. Validation of candidate reference methods based on ion chromatography for determination of total sodium, potassium, calcium and magnesium in serum through comparison with flame atomic emission and absorption spectrometry . Clin Biochem . 1996; 29 ( 6 ):501–508. [PubMed] [Google Scholar]
[432] Pullan NJ, Thurston V, Barber S. Evaluation of an inductively coupled plasma mass spectrometry method for the analysis of sweat chloride and sodium for use in the diagnosis of cystic fibrosis . Ann Clin Biochem . 2013; 50 ( Pt 3 ):267–270. [PubMed] [Google Scholar]
[433] Goulet EDB, Asselin A, Gosselin J, et al. Measurement of sodium concentration in sweat samples: comparison of 5 analytical techniques . Appl Physiol Nutr Metab . 2017; 42 ( 8 ):861–868. [PubMed] [Google Scholar]
[434] Sato K, Sato F. Nonisotonicity of simian eccrine primary sweat induced in vitro . Am J Physiol . 1987; 252 ( 6 Pt 2 ):R1099–105. [PubMed] [Google Scholar]
[435] Dill DB, Hall FG, Van Beaumont W. Sweat chloride concentration: sweat rate, metabolic rate, skin temperature, and age . J Appl Physiol . 1966; 21 ( 1 ):99–106. [PubMed] [Google Scholar]
[436] Gordon RS Jr., Cage GW. Mechanism of water and electrolyte secretion by the eccrine sweat gland . Lancet . 1966; 1 ( 7449 ):1246–1250. [PubMed] [Google Scholar]
[437] Buono MJ, Stone M, Cannon DT. Leaching from the stratum corneum does not explain the previously reported elevated potassium ion concentration in sweat . J Basic Clin Physiol Pharmacol . 2016; 27 ( 2 ):171–173. [PubMed] [Google Scholar]
[438] Sato K. Electrochemical driving forces for K+ secretion by rat paw eccrine sweat gland . Am J Physiol . 1980; 239 ( 3 ):C90–7. [PubMed] [Google Scholar]
[439] Sato K. Sweat induction from an isolated eccrine sweat gland . Am J Physiol . 1973; 225 ( 5 ):1147–1152. [PubMed] [Google Scholar]
[440] Green JM, Bishop PA, Muir IH, et al. Effects of high and low blood lactate concentrations on sweat lactate response . Int J Sports Med . 2000; 21 ( 8 ):556–560. [PubMed] [Google Scholar]
[441] Alvear-Ordenes I, García-López D, De Paz JA, et al. Sweat lactate, ammonia, and urea in rugby players . Int J Sports Med . 2005; 26 ( 8 ):632–637. [PubMed] [Google Scholar]
[442] Fellmann N, Grizard G, Coudert J. Human frontal sweat rate and lactate concentration during heat exposure and exercise . J Appl Physiol Respir Environ Exerc Physiol . 1983; 54 ( 2 ):355–360. [PubMed] [Google Scholar]
[443] Patterson MJ, Galloway SDR, Nimmo MA. Effect of induced metabolic alkalosis on sweat composition in men . Acta Physiol Scand . 2002; 174 ( 1 ):41–46. [PubMed] [Google Scholar]
[444] Lemon PW, Yarasheski KE, Dolny DG. Validity/reliability of sweat analysis by whole-body washdown vs. regional collections . J Appl Physiol . 1986; 61 ( 5 ):1967–1971. [PubMed] [Google Scholar]
[445] Colombani P, Spati S, Spleiss C, et al. Exercise-induced swet nitrogen excretion: evaluation of a regional collection method using gauze pads . Eur J Nutr . 1997; 36 :237–243. [PubMed] [Google Scholar]
[446] Moyer J, Wilson D, Finkelshtein I, et al. Correlation between sweat glucose and blood glucose in subjects with diabetes . Diabetes Technol Ther . 2012; 14 ( 5 ):398–402. [PubMed] [Google Scholar]
[447] Lee H, Song C, Hong YS, et al. Wearable/disposable sweat-based glucose monitoring device with multistage transdermal drug delivery module . Sci Adv . 2017; 3 ( 3 ):e1601314. [PMC free article] [PubMed] [Google Scholar]
[448] Silvers S, Forster W, Talbert GA. Simultaneous study of the constituents of the sweat, urine and blood, also gastric acidity and other manifestations resulting from sweating. VI. Sugar . Am J Physiol . 1928; 84 :577–582. [Google Scholar]
[449] Jajack A, Brothers M, Kasting G, et al. Enhancing glucose flux into sweat by increasing paracellular permeability of the sweat gland . PLoS One . 2018; 13 ( 7 ):e0200009. [PMC free article] [PubMed] [Google Scholar]
[450] Page CO Jr., Remington JS. Immunologic studies in normal human sweat . J Lab Clin Med . 1967; 69 ( 4 ):634–650. [PubMed] [Google Scholar]
[451] Heikenfeld J, Jajack A, Feldman B, et al. Accessing analytes in biofluids for peripheral biochemical monitoring . Nat Biotechnol . 2019; 37 :407–419. [PubMed] [Google Scholar]
[452] Cizza G, Marques AH, Eskandari F, et al. Elevated neuroimmune biomarkers in sweat patches and plasma of premenopausal women with major depressive disorder in remission: the POWER study . Biol Psychiatry . 2008; 64 ( 10 ):907–911. [PMC free article] [PubMed] [Google Scholar]
[453] Marques-Deak A, Cizza G, Eskandari F, et al. Measurement of cytokines in sweat patches and plasma in healthy women: validation in a controlled study . J Immunol Methods . 2006; 315 ( 1–2 ):99–109. [PubMed] [Google Scholar]
[454] Dai X, Okazaki H, Hanakawa Y, et al. Eccrine sweat contains IL-1alpha, IL-1beta and IL-31 and activates epidermal keratinocytes as a danger signal . PLoS One . 2013; 8 ( 7 ):e67666. [PMC free article] [PubMed] [Google Scholar]
[455] Reitamo S, Anttila HS, Didierjean L, et al. Immunohistochemical identification of interleukin I alpha and beta in human eccrine sweat-gland apparatus . Br J Dermatol . 1990; 122 ( 3 ):315–323. [PubMed] [Google Scholar]
[456] Yokozeki H, Hibino T, Takemura T, et al. Cysteine proteinase inhibitor in eccrine sweat is derived from sweat gland . Am J Physiol . 1991; 260 ( 2 Pt 2 ):R314–20. [PubMed] [Google Scholar]
[457] Horie N, Yokozeki H, Sato K. Proteolytic enzymes in human eccrine sweat: a screening study . Am J Physiol . 1986; 250 ( 4 Pt 2 ):R691–8. [PubMed] [Google Scholar]
[458] Imbeault P, Ravanelli N, Chevrier J. Can POPs be substantially popped out through sweat? Environ Int . 2018; 111 :131–132. [PubMed] [Google Scholar]
[459] Davis SL, Wilson TE, Vener JM, et al. Pilocarpine-induced sweat gland function in individuals with multiple sclerosis . J Appl Physiol (1985) . 2005; 98 ( 5 ):1740–1744. [PubMed] [Google Scholar]
[460] Castle PC, Kularatne BP, Brewer J, et al. Partial heat acclimation of athletes with spinal cord lesion . Eur J Appl Physiol . 2013; 113 ( 1 ):109–115. [PubMed] [Google Scholar]
[461] Yaggie JA, Niemi TJ, Buono MJ. Adaptive sweat gland response after spinal cord injury . Arch Phys Med Rehabil . 2002; 83 ( 6 ):802–805. [PubMed] [Google Scholar]
[462] Price MJ. Thermoregulation during exercise in individuals with spinal cord injuries . Sports Med . 2006; 36 ( 10 ):863–879. [PubMed] [Google Scholar]
[463] Price MJ, Campbell IG. Thermoregulatory responses of paraplegic and able-bodied athletes at rest and during prolonged upper body exercise and passive recovery . Eur J Appl Physiol Occup Physiol . 1997; 76 ( 6 ):552–560. [PubMed] [Google Scholar]
[464] Ono E, Murota H, Mori Y, et al. Sweat glucose and GLUT2 expression in atopic dermatitis: implication for clinical manifestation and treatment . PLoS One . 2018; 13 ( 4 ):e0195960. [PMC free article] [PubMed] [Google Scholar]
[465] Lear W, Kessler E, Solish N, et al. An epidemiological study of hyperhidrosis . Dermatol Surg . 2007; 33 ( 1 Spec No ):S69–75. [PubMed] [Google Scholar]
[466] Luetkemeier MJ, Hanisko JM, Aho KM. Skin Tattoos Alter Sweat Rate and Na+ Concentration . Med Sci Sports Exerc . 2017; 49 ( 7 ):1432–1436. [PubMed] [Google Scholar]
[467] Pizzey FK, Parupia IM, Allen DR, et al. Attenuated sweating responses in tattooed skin during a passive whole-body heat stress . Faseb J . 2017; 31 ( 1 Supplement ):lb746. [Google Scholar]
[468] Cotton DW, Kuypers BR. Thermal induced sweating in tattooed skin . Dermatologica . 1970; 141 ( 3 ):252–254. [PubMed] [Google Scholar]
Articles from Temperature: Multidisciplinary Biomedical Journal are provided here courtesy of Taylor & Francis