Endocrine system 1: overview of the endocrine system and hormones
The endocrine system comprises glands and tissues that produce hormones for regulating and coordinating vital bodily functions. This article, the first in an eight-part series, is an overview of the system
Abstract
The endocrine system is made up of glands and tissues that produce and secrete hormones to regulate and coordinate vital bodily functions. This article – the first in an eight-part series on the anatomy and physiology of the endocrine system – explores the nature of endocrine glands and tissues, and the role of hormones as chemical signals that are carried in the blood. It also highlights the varying roles of hormones in regulating and coordinating physiological processes, as well as maintaining homoeostasis in the body.
Citation: Knight J (2021) Endocrine system I: overview of the endocrine system and hormones. Nursing Times [online]; 117: 5, 38-42.
Author: John Knight is associate professor in biomedical science, College of Human and Health Sciences, Swansea University.
- This article has been double-blind peer reviewed
- Scroll down to read the article or download a print-friendly PDF here (if the PDF fails to fully download please try again using a different browser)
- Assess your knowledge and gain CPD evidence by taking the Nursing Times Self-assessment test
- Click here to see other articles in this series
Introduction
The endocrine system is a series of glands and tissues that produce and secrete hormones, which are used by the body to regulate and coordinate vital bodily functions, including growth and development, metabolism, sexual function and reproduction, sleep and mood. This article – the first in an eight-part series on the anatomy and physiology of the endocrine system – provides an overview of the system, focusing on endocrine glands and tissues, and the role of hormones as chemical signals that are bloodborne. It also explains the diverse roles of hormones in regulating and coordinating physiological processes, and maintaining homoeostatic balance in the body.
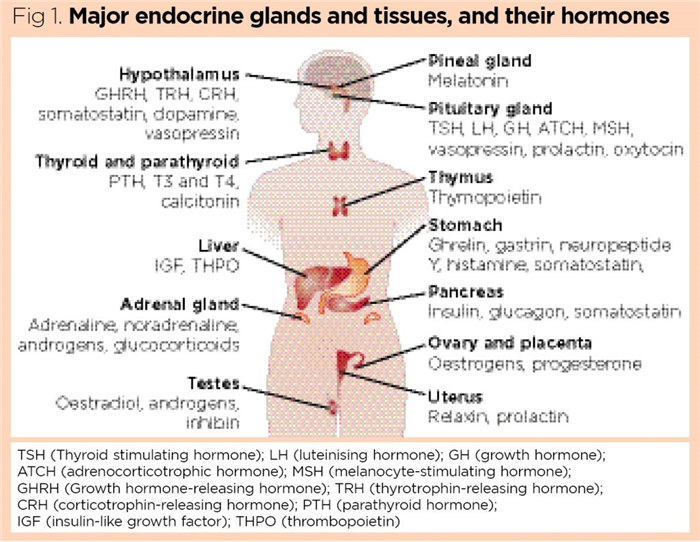
The endocrine system (Fig 1) is incredibly complex: it consists of dedicated, specialised endocrine glands – such as the thyroid, parathyroids and adrenal glands – together with tissues such as fat (adipose tissue) and bone that have a secondary endocrine function and also secrete a range of hormones. It has been suggested that the microbial biome (the diverse plethora of micro-organisms colonising the human body) also functions as a “virtual endocrine organ”, secreting a cocktail of chemical signals that further influences human physiology (O’Callaghan et al, 2016).
Endocrine and exocrine glands
By definition, all glandular tissues produce secretions. Most glandular structures are epithelial in origin, and many are folded and organised into recognisable glands with a central duct. Glands possessing a duct are exocrine glands (Fig 2); the duct acts as a conduit into which secretions are released before being carried away to their sites of action. Exocrine glands include many of the digestive glands in the gut, sweat glands in the skin and mucus-producing glands in the mucous membranes of the mouth and reproductive tracts.
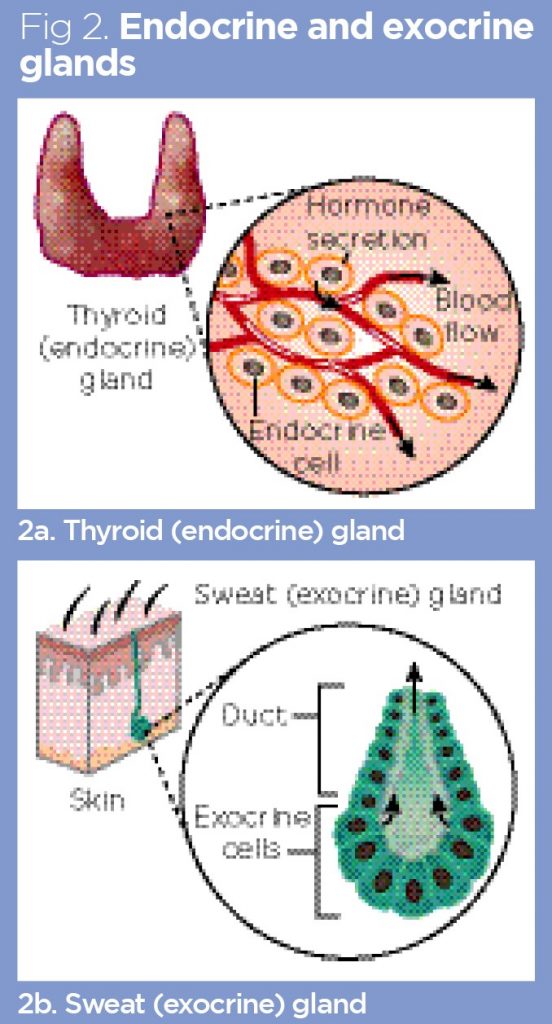
In contrast, endocrine glands have no duct, but release their secretions, called hormones, directly into the blood (Fig 2). For this reason, most endocrine glands are highly vascularised, and many of their component cells are in direct contact with blood capillaries. This close association with blood vessels facilitates the direct release of hormones into the blood and allows the blood to be continuously monitored for physiological changes that can initiate hormone release. As an example of this, the insulin-producing cells of the pancreas will release insulin when they detect an increase in blood–glucose concentration after the consumption of carbohydrate-rich food.
The highly vascular nature of endocrine glands also allows for the delivery of signals (usually other hormones) from other glands to regulate release of their own hormones. For example, the thyroid gland releases hormones that regulate metabolism, such as thyroxine, in response to the thyroid-stimulating hormone, which is produced by the anterior pituitary gland.
The major endocrine glands
Fig 1 shows the position of the major endocrine glands in the body; however, it is important to be aware that many other organs and tissues have a secondary endocrine function, including the heart, kidneys, bone and adipose tissues (Knight et al, 2020; Moser and van der Eerden, 2019).
The hypothalamus
The hypothalamus is a vital region of the brain, which plays an important role in:
- Thermoregulation;
- Behavioural and emotional responses;
- Regulation of appetite;
- Coordination of the autonomic nervous system;
- Generating a range of hormones that regulate the activity of endocrine glands.
Indeed, the hypothalamus can be thought of as the key crossover point between the nervous system and the endocrine system.
The pituitary gland
The pituitary gland is a pea-sized structure, typically weighing around 500mg; it is located at the base of the brain, just behind the nasal cavity, where it is protected by the sphenoid bone of the skull (Ganapathy and Tadi, 2020). It has two major regions:
- The posterior (back portion) –essentially, an extension of the hypothalamus, the posterior of the pituitary gland stores and concentrates two neuropeptide hormones called anti-diuretic hormone (ADH) and oxytocin, which are produced by the neurons (nerve cells) of the hypothalamus. ADH helps regulate fluid balance and blood pressure, while oxytocin – among other things – initiates parturition (childbirth).
- The anterior (front portion) – this develops from the epithelial tissues in the roof of the embryonic oral cavity, which bulges up into the skull, fusing with the posterior pituitary. It produces several key hormones such as somatotropin (growth hormone) and melanocyte-stimulating hormone, which helps to regulate skin pigmentation. The anterior pituitary also produces several stimulating hormones that control the release of hormones from other endocrine glands. As an example of this, adrenocorticotropic hormone regulates the release of the long-term stress hormone, cortisol, from the adrenal cortex.
As the pituitary gland regulates hormone release from other endocrine glands, it is often referred to as the ‘master’ gland. This is something of a misnomer as the release of stimulating hormones from the pituitary gland is, itself, under the control of hormones produced by the hypothalamus; this will be explored in Part 2.
Thyroid gland and associated parathyroids
The thyroid is a bilobed (two-lobed) organ that resembles a bow tie in shape; it typically weighs 25-30g and is located just below the larynx (Dorion, 2017). The thyroid itself has two major populations of endocrine cells:
- Follicular cells – these produce the iodine-containing hormones triiodothyronine (T3) and tetraiodothyronine (T4, also known as thyroxine), which regulate the body’s metabolism;
- Parafollicular cells – these produce the hormone calcitonin, which helps to regulate blood–calcium concentration.
The parathyroid glands are found embedded in the posterior portion of the thyroid gland. Most people have four parathyroid glands (explored in Part 3); these produce parathyroid hormone, which works antagonistically to calcitonin during calcium homoeostasis.
Pancreas
The pancreas is a vital organ in both the digestive and the endocrine systems; residing in the U-shaped loop of the duodenum, it is typically 14-23cm in length and weighs around 100g (Longnecker, 2021).
The endocrine portions of the pancreas are known as the islets of Langerhans, which are small islands of glandular tissue found throughout the structure of the pancreas. The pancreatic islets contain several types of endocrine cells, including:
These two hormones – glucagon and insulin – play a key role in regulating blood–glucose concentration, which will be discussed in the section on homoeostasis later in this article.
Adrenal glands
There are two adrenal glands – one above each kidney. They are roughly triangular in shape, around 3cm in width and each weighs 4-6g (Lack and Paal, 2020). Adrenal glands have two major regions:
- Adrenal cortex (outer region) – this produces steroid hormones, including the long-term stress hormone cortisol, aldosterone (which regulates the levels of sodium and potassium in the blood) and a group of testosterone-like hormones called androgens;
- Adrenal medulla (inner region) – this produces adrenaline (epinephrine) and noradrenaline (norepinephrine). These ‘fight-or-flight’ hormones – that are usually produced when a person is under threat, afraid or excited – function primarily to activate the sympathetic branch of the autonomic nervous system and prepare the body for immediate action.
Ovaries and testes
The ovaries are the primary reproductive organs in females, responsible for producing ova. Mature ovaries are fairly irregular, lumpy and almond shaped, typically 3-5cm long and weigh 5-8g, although they tend to decrease in size in later life (Wallace and Kelsey, 2004). Ova develop in fluid-filled sacs called follicles; as follicles enlarge, they release oestrogen, the female sex hormone that promotes the thickening of the uterine lining (endometrium).
Once a follicle ruptures and releases its mature ovum into the fallopian tube during ovulation, the remnants of the follicle collapse into a structure called the corpus luteum (yellow body). This produces the second major female sex hormone, progesterone, which prepares the endometrium for implantation of a fertilised ovum (zygote) and, subsequently, maintains the integrity of the endometrial lining, should implantation occur.
The testes (testicles) are the paired primary reproductive organs in males, responsible for producing spermatozoa. They are oval shaped and, in adult males, are typically 4.5-5.1cm long and weigh 15-19g (Silber, 2018). Each testis contains a specialised group of endocrine cells called interstitial cells, which produce the male sex hormone testosterone. This is an anabolic steroid produced in greater amounts during puberty, when it promotes muscle development, growth of facial and body hair, and expansion of the larynx, leading to a deepening of the voice.
“It has been suggested that the microbial biome (the diverse micro-organisms colonising the body) also functions as a virtual endocrine organ”
Hormones as chemical signals
Hormones are traditionally defined as chemical signals, transported to their target tissues in the blood; today, however, that definition is often expanded to include all chemical messengers that bind to target cells with high affinity. So far, more than 100 hormones have been identified in the human body, and this rises to more than 200 if hormone-like substances are included (Silver and Kriegsfeld, 2001).
Hormones exert their physiological effects by binding to specific receptors associated with their target cells (Fig 3). Many drugs have been designed to target these receptor sites, either to mimic the actions of hormones (for example, in the case of a hormone deficiency such as hypothyroidism, which is treated with levothyroxine) or to act as competitive antagonists to physically block the receptor, preventing the natural hormone from binding and exerting its effect. Hormones can be broadly divided into three major classes:
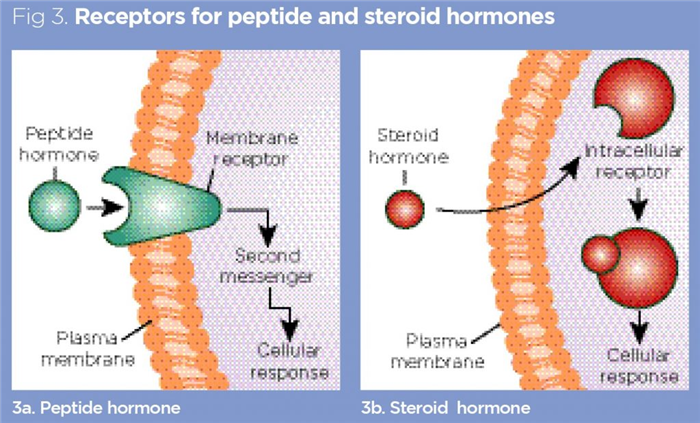
- Peptide hormones;
- Steroid hormones;
- Amino acid-derived hormones.
Peptide hormones
These are the largest hormones, with relatively high molecular weights. They are proteinaceous chemical signals, comprising chains of amino acids of varying lengths. Examples include:
Some peptide hormones are initially produced as inactive forms called prohormones; a good example is insulin, which is first synthesised as a much larger molecule, called proinsulin, and then cleaved into its active, shorter form before being released into the blood.
Peptide hormones tend to exert their effects by binding to receptors on the surface of the plasma membranes of target cells, as shown in Fig 3. This triggers a variety of transmembrane events, leading to the production of second messengers (such as cyclic adenosine monophosphate), which, subsequently, initiate the desired effect of the hormone in the target cell (Foster et al, 2019).
Steroid hormones
Steroid hormones are lipids (fats), mostly derived directly from cholesterol, which acts as a precursor molecule for steroid biosynthesis. Examples include:
As steroid hormones are lipids, they rapidly diffuse across the phospholipid bilayer of their target cell membranes (Fig 3) and exert their effects by binding to receptors in the cytoplasm or nucleus (Ozawa, 2006). Steroid hormones tend to precipitate their desired effects by modulating the activity of particular genes in cells.
Amino acid-derived hormones
These are synthesised from amino acids, so are small molecules with low molecular weights. Examples include:
- Adrenaline (epinephrine), derived from tyrosine;
- Thyroid hormones thyroxine T4 and T3, derived from tyrosine;
- Melatonin (which helps to regulate sleep), derived from tryptophan (Kleine and Rossmanith, 2016).
Like peptide hormones, some amino acid-derived hormones, such as adrenaline, bind to receptors on the surface of target-cell plasma membranes. Others however, such as T3 from the thyroid, cross the plasma membranes of their target cells and bind to receptors inside the cell in a similar manner to steroid hormones.
Locally acting hormones: autocrine and paracrine
As well as the hormones secreted by the major endocrine glands, there are various locally acting hormone-like substances. These are usually released into the interstitial fluid (the thin film of tissue fluid surrounding most cells) and exert their effects in the local vicinity.
Autocoids are chemical signals released by a cell that exert their effects on that same cell; paracrine signals act more widely, affecting neighbouring cells in the immediate vicinity (Alberts et al, 2015). These locally acting hormones – both autocrine and paracrine – are usually rapidly broken down before they can enter the wider circulation. Good examples are the eicosanoids, a large family of lipid-derived molecules, which include the prostaglandins, thromboxanes, leukotrienes and lipoxins (O’Donnell et al, 2009).
Prostaglandins and the fever response
Fever (pyrexia) is commonly associated with infection. When phagocytic leukocytes (white blood cells) such as monocytes enter sites of infection and begin to trap and kill pathogens, they release a cytokine (a signalling chemical that is produced by immune cells) called interleukin-1 (IL-1). IL-1 is a small peptide that circulates in the blood before binding to receptors on cells in the hypothalamus – the region of the brain containing the thermoregulatory centre that is responsible for controlling body temperature, which usually has a set point of around 37°C (Knight et al, 2020).
Once IL-1 has binded to its receptor, the enzyme cyclooxygenase (COX) is activated, leading to the production of the eicosanoid, prostaglandin E2 (PGE2); this locally acting signal shifts the set point of the hypothalamus upwards (typically to around 38°C-39°C), leading to fever (Eskilsson et al, 2017).
Fever is a useful response during infection as it can slow the replication of pathogens, while simultaneously speeding up and enhancing pathogen killing by leukocytes. However, fever also takes enzymes in the body cells outside of their normal optimal temperature of 37°C, slowing the biochemical reactions that are necessary for life. This can cause people to experience malaise and feel generally unwell until the infection is dealt with and the temperature can return to normal.
If fever becomes extremely high (≥40°C), there is an increased risk of febrile convulsions. Antipyretic drugs – which include many common non-steroidal anti-inflammatory drugs (NSAIDs), such as aspirin – may be given to reduce the fever. NSAIDs work, primarily, by inhibiting the activity of the enzyme COX, thereby preventing the production of PGE2 and shifting upwards the set point of the thermoregulatory centre.
If a patient’s fever needs to be reduced, it is common practice to combine the use of antipyretic drugs and interventions such as reducing bed linen – for example, air-circulating or water-circulating blankets or hydrogel-coated water-circulating pads can also be used. There is no evidence that fans help with temperature regulation and should be avoided as they can increase the risk of shivering (Doyle and Schortgen, 2016).
The endocrine system and homoeostasis
An average adult human with a weight of 70kg is thought to comprise around 30-40 trillion cells (Sender et al, 2016). For each cell to function effectively, it must be maintained at the correct temperature and pH, and provided with a steady stream of nutrients and oxygen. At the same time, the local environment of each cell needs any waste metabolites, such as carbon dioxide and urea, to be efficiently removed.
Homoeostasis can be broadly defined as the ability to maintain a relatively stable internal environment; it is essential to good health and survival (Modell et al, 2015). A multitude of variables in the body are susceptible to continual and significant fluctuation, and most of the major organ systems of the body are dedicated to keeping these variables within their normal physiological ranges.
The internal biochemical processes necessary for life are primarily driven by biological catalysts known as enzymes, which generally fall into two categories:
- Anabolic enzymes – these are responsible for building molecules in the body. For example, DNA polymerase builds new molecules of DNA necessary for cell division and growth, while glycogen synthase takes single molecules of glucose and polymerises them (links them together) to form long, branching chains of glycogen (animal starch), which is stored in large amounts in liver and muscle;
- Catabolic enzymes – these break down molecules and include the enzymes of the digestive tract, which digest the macromolecules (large, complex molecules) of food into simple components that can be absorbed and used by the body. Other key catabolic enzymes are those involved in cellular respiration, in which sugars are metabolised (usually in the presence of oxygen) to release the energy necessary for life.
Anabolic and catabolic enzymes can only function efficiently in narrow ranges of temperature and pH; they also require a steady supply of the substrate molecules on which they act (Puri, 2018). As an example, for aerobic cellular metabolism to occur, the respiratory enzymes in cells require a steady stream of glucose and oxygen.
The homoeostatic mechanisms that ensure a stable environment in the body rely on a process called negative feedback, which is discussed below.
Set points, negative feedback and the role of hormones
For each variable in the human body, there is a hypothetical ideal value – the set point. As an example, the set point for glucose is around 5mmol/L (Fig 4); at 5mmol/L, human cells are supplied with a steady supply of glucose, which can be used to release energy during cellular respiration.
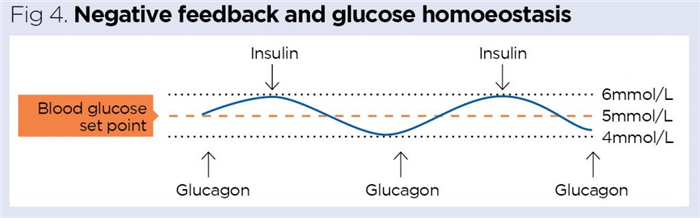
The body strives to maintain variables as close to their set points as possible using negative-feedback mechanisms. During negative feedback, any deviations from the set point are resisted and minimised, allowing a variable to be constrained within a narrow, normal physiological range. If blood–glucose concentration is measured throughout the day, it would be expected to fluctuate around its set point. As an example, after exercise, blood–glucose concentration typically falls as glucose is used to provide energy for muscle contraction; conversely, after a carbohydrate-rich meal or snack (such as a chocolate bar), the blood–glucose level rises.
Hormones frequently play major roles in negative feedback and often work together in antagonistic pairs. Fig 4 shows that when blood–glucose concentration rises, the hormone insulin is released; this promotes glucose uptake by the cells of the body and the blood–glucose level drops. Conversely, if blood–glucose concentration falls, the hormone glucagon is released; this stimulates the release of stored glucose from the liver, which causes blood glucose to rise again. The two pancreatic hormones, insulin and glucagon, work antagonistically to each other to effectively constrain the blood–glucose concentration in its normal physiological range of 4-6mmol/L (Knight et al, 2020).
Effects of variables outside of their normal range
One in 14 people in the UK has the chronic metabolic disease, diabetes mellitus, which means they no longer produce insulin (type 1) or become resistant to its effects (type 2). Without an effective insulin response, blood–glucose concentration will rise markedly above its normal physiological range. Some undiagnosed patients with diabetes can have seriously high blood–glucose concentrations of >33mmol/L requiring immediate treatment. Elevated blood glucose is called hyperglycaemia and is the key clinical feature of diabetes.
Many patients with diabetes inject insulin to manage and normalise their blood–glucose levels. On occasion, some may inject too much insulin or eat insufficient carbohydrate so their blood–glucose concentration falls far below its normal physiological range; this is called hypoglycaemia and can be extremely dangerous. When pronounced, hypoglycaemia can lead to mental impairment, behavioural changes, unconsciousness, coma and potentially death (Mukherjee et al, 2011).
The example of hyperglycaemia and hypoglycaemia shows how, when a variable is taken outside of its normal range for any protracted length of time, it is detrimental to health and leads to pathology (disease states); both hyperglycaemia and hypoglycaemia are frequently encountered in poorly managed diabetes.
Conclusion
This article has provided a general overview of the nature of hormones, along with the major endocrine glands and their importance in regulating and coordinating vital bodily functions. Each of the major endocrine glands and their hormonal secretions will be be examined in greater detail later in the series; part 2 focuses on the hypothalamus and pituitary gland.
Key points
- The endocrine system comprises glands and tissues that secrete hormones to regulate and coordinate vital functions in the body
- Endocrine glands differ from exocrine glands by releasing their secretions directly into the bloodstream, rather than a central duct
- Endocrine glands’ highly vascular nature allows variables in the blood to be monitored continuously and appropriate hormones to be rapidly released into the circulation
- Hormones exert their physiological effects by binding to specific receptors associated with their target cells
- Hormones regulate physiological processes and are key to maintaining homoeostatic balance in the body
Also in this series

- Test your knowledge with Nursing Times Self-assessment after reading this article. If you score 80% or more, you will receive a personalised certificate that you can download and store in your NT Portfolio as CPD or revalidation evidence.
- Take the Nursing Times Self-assessment for this article
Alberts B et al (2015) The Molecular Biology of the Cell. Garland Science.
Dorion D (2017) Thyroid Anatomy. reference.medscape.com, 30 November.
Doyle JF, Schortgen F (2016) Should we treat pyrexia? And how do we do it? Critical Care; 20, 303.
Eskilsson A et al (2017) Immune-induced fever Is dependent on local but not generalized prostaglandin E2 synthesis in the brain. Journal of Neuroscience; 37: 19, 5035-5044.
Foster SR et al (2019) Discovery of human signaling systems: pairing peptides to G protein-coupled receptors. Cell; 179: 4, 895-908.
Ganapathy MK, Tadi P (2020) Anatomy, Head and Neck, Pituitary Gland. StatPearls.
Kleine B, Rossmanith WG (2016) Hormones and the Endocrine System. Springer.
Knight J et al (2020) The Endocrine System. In: Understanding Anatomy and Physiology in Nursing. Sage.
Lack EE, Paal E (2020) Adrenal glands. In: Cheng L et al (eds) Urologic Surgical Pathology. Elsevier.
Longnecker DS (2021) Anatomy and histology of the pancreas. The Pancreapedia: Exocrine Pancreas Knowledge Base. doi: 10.3998/panc.2021.01.
Modell H et al (2015) A physiologist’s view of homoeostasis. Advances in Physiology Education; 39: 4, 259-266.
Moser SC, van der Eerden BCJ (2019) Osteocalcin – a versatile bone-derived hormone. Frontiers in Endocrinology; 9: 794.
Mukherjee E et al (2011) Endocrine and metabolic emergencies: hypoglycaemia. Therapeutic Advances in Endocrinology and Metabolism; 2: 2, 81-93.
O’Callaghan TF et al (2016) The gut microbiome as a virtual endocrine organ with implications for farm and domestic animal endocrinology. Domestic Animal Endocrinology; 56: S44-S55.
O’Donnell VB et al (2009) Eicosanoids: generation and detection in mammalian cells. Methods in Molecular Biology; 462: 5-23.
Ozawa H (2006) Steroid hormones, their receptors and neuroendocrine system. Journal of Nippon Medical School; 72: 6, 316-325.
Puri D (2018) Textbook of Medical Biochemistry. Elsevier.
Sender R et al (2016) Revised estimates for the number of human and bacteria cells in the body. PLoS Biology; 14: 8, e1002533.
Silber S (2018) Fundamentals of Male Infertility. Springer.
Silver R, Kriegsfeld LJ (2001) Hormones and Behaviour. Wiley Online Library.
Wallace WH, Kelsey TW (2004) Ovarian reserve and reproductive age may be determined from measurement of ovarian volume by transvaginal sonography. Human Reproduction; 19: 7, 1612-1617.