Endocrine system 5: the functions of the pineal and thymus glands
The endocrine system comprises glands and tissues that produce hormones for regulating and coordinating vital bodily functions. This article, the fifth in an eight-part series, looks at the pineal and thymus glands
Abstract
The endocrine system consists of glands and tissues that produce and secrete hormones to regulate and coordinate vital bodily functions. This article, the fifth in an eight-part series on the endocrine system, explores the anatomy and physiology of the pineal and thymus glands, and describes how they regulate and coordinate vital physiological processes in the body through hormonal action.
Citation: Knight J et al (2021) Endocrine system 5: pineal and thymus glands. Nursing Times [online]; 117: 9, 54-58.
Authors: John Knight is associate professor in biomedical science; Zubeyde Bayram-Weston is senior lecturer in biomedical science; Maria Andrade is honorary associate professor in biomedical science; all at College of Human and Health Sciences, Swansea University.
- This article has been double-blind peer reviewed
- Scroll down to read the article or download a print-friendly PDF here (if the PDF fails to fully download please try again using a different browser)
- Click here to see other articles in this series
Introduction
The endocrine system comprises glands and tissues that produce hormones to regulate and coordinate vital bodily functions. This fifth article in an eight-part series examines the anatomy, physiology and function of the pineal and thymus glands. Two of the smallest endocrine glands, these are known to undergo significant age-associated changes that influence human physiology.
Pineal gland: location and histology
The pineal gland, also known as the pineal body, is a neuroendocrine gland found towards the centre of the brain. It is located medially between the two cerebral hemispheres (Fig 1) and is attached to the rear of the third ventricle by a small pineal stalk.
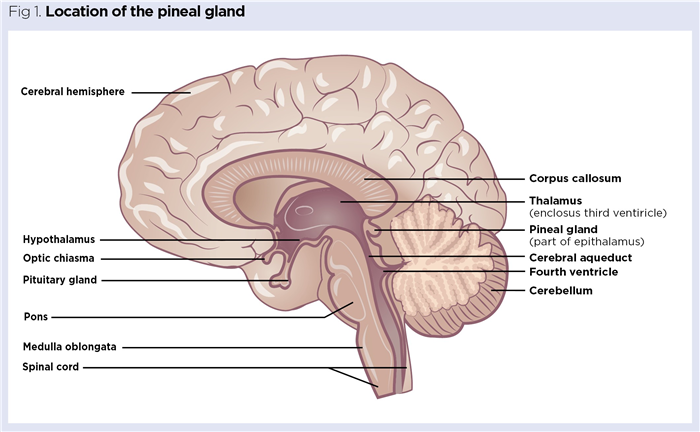
The term ‘pineal’ refers to the structure of the gland, which often resembles a pine cone. However, there is much variation in shape, with many human pineal glands being pea-shaped or fusiform (tapering at both ends) (Afroz et al, 2014). The average adult pineal gland is a tiny brown structure, typically 5-8mm long, that weighs around 150mg. It is highly vascularised, with a rich blood flow of around 4ml/min/g, second only to that of the kidneys. Unlike most other brain components, the pineal gland is found outside of the neuroprotective blood brain barrier (Chlubek and Sikora, 2020). It increases in size throughout early childhood and becomes fully developed at around age 5 to 7 years (Patel et al, 2020).
Histologically, 95% of the cells of the pineal gland are the pinealocytes, the rest are supporting cells (Ibañez Rodriguez et al, 2016). The primary role of pinealocytes is to synthesise and secrete the chronobiotic hormone melatonin, which plays a key role in synchronising the body’s biological rhythms. Structurally, pinealocytes are believed to be related to the photosensitive rods and cones of the retina; although, in humans, they only have an indirect response to light, as detected at the retina, through a series of complex neural pathways (Zhao et al, 2019).
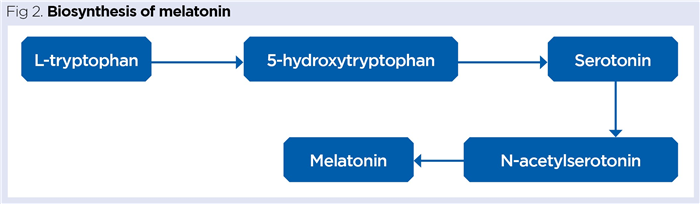
Pinealocytes take up the essential amino acid tryptophan, which is enzymatically converted into the neurotransmitter serotonin and then into melatonin (Fig 2).
The suprachiasmatic nucleus as circadian clock
Melatonin secretion is governed by light levels perceived by the retina. Action potentials (nerve impulses) generated from retinal rods and cones are relayed to the visual cortex of the brain via the optic nerves. These cross over each other at the optic chiasm (Fig 3), above which is a specialised collection of around 10,000 neurons called the suprachiasmatic nucleus (SCN).
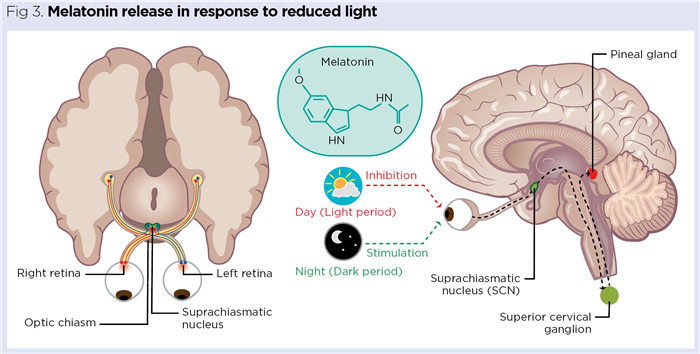
The SCN is a vital part of the hypothalamus and functions as the body’s biological clock (circadian master clock). Neurons in the SCN have an inherent 24-hour rhythm synchronised to cues in light intensity detected by the retina (Challet, 2015). The major trigger for melatonin release is reduced light, with neural output from the SCN stimulating the pineal gland via sympathetic nerves originating in the superior cervical ganglion in the neck (Fig 3). The SCN establishes the basic circadian rhythms that influence many physiological processes, including the sleep/wake cycle, body temperature, blood pressure and appetite (Douma and Gumz, 2018).
Melatonin secretion tends to begin at night, around two hours before sleep (typically around 10pm); it maximises in the early hours of the morning (between 2am and 4am), with plasma concentrations typically reaching 80-150 picograms (pg)/ml (Chlubek and Sikora, 2020). This usually corresponds to the period of deepest sleep. As light gradually returns, levels decrease again, with secretion usually ceasing around 8am.
Role of melatonin
Initially, melatonin is released into the densely arranged local blood vessels of the pineal gland and the cerebrospinal fluid of the third ventricle; it then enters the general circulation and is distributed systemically around the body (Zisapel, 2018). It is also synthesised and released from a wide range of other human organs, tissues and cells, including the retina, bone marrow, lymphocytes, platelets, skin and gastrointestinal tract (Tordjman et al, 2017). Melatonin circulates in the plasma and, like most hormones, exerts its effects by binding to specific receptors. These are widespread throughout the human body; few tissues are thought to be devoid of melatonin receptors (Emet et al, 2016; Omar and Saba, 2010).
Sleep/wake cycle
It has long been established that melatonin is an important regulator of sleep in diurnal species such as humans. Sleep, which accounts for around a third of our lives, is recognised as a highly orchestrated neurochemical process, involving both arousal and sleep-promoting regions of the brain that are influenced by a variety of chemical signals and cues (Zisapel, 2018). It is essential to both physical and psychological health, and facilitates a significant reduction in external sensory input while simultaneously allowing unconscious:
- Processing of information acquired while awake;
- Consolidation of memories;
- Clearance of potentially harmful metabolites from neural tissues.
The exact mechanisms by which melatonin influences sleep remain unclear, but it is recognised that it reduces sleep latency (time taken to fall asleep), while improving sleep quality and duration, and reducing fragmented sleep (Grima et al, 2017).
Insomnia and sleep disruption
Insomnia, defined as a difficulty in falling or staying asleep, is estimated to affect around one in three people in the UK. Melatonin has been shown to be a non-addictive substance of therapeutic value in treating patients experiencing insomnia. Exogenous melatonin prolongs sleep, improves sleep efficiency, enhances sleep quality and subsequently, when awake, improves functional performance and mental acuity (Grima et al 2017).
The blue light emitted from the screens of electronic devices (such as TVs, computers and mobile phones) is known to suppress melatonin biosynthesis and secretion. Exposure to such displays for at least 30 minutes before sleep is associated with increased sleep latency, poor sleep quality, sleep disruption and increased daytime sleepiness (Rafique et al, 2020). Reducing blue-light exposure, particularly in the evening before going to bed, has been demonstrated to improve sleep quality (Shechter et al, 2018) and some devices have settings or apps to restrict the amount of blue light emitted. Spectacles with blue-light filters are also available, which can effectively block the amount of blue light reaching the retina, thereby enhancing melatonin release (Pateras, 2020).
Antioxidant
Melatonin is a powerful antioxidant, acting as an efficient scavenger of free radicals (for example, superoxide anions) that could, otherwise, inflict significant cellular damage and denature proteins and nucleic acids. This is of particular importance in the mitochondria, where cellular respiration can generate large quantities of free radicals capable of damaging mitochondrial DNA and potentially causing genetic mutation (Cipolla-Neto and Amaral, 2018).
“Melatonin appears to be so effective in inhibiting the proliferation of certain tumour cells that the pineal gland has been routinely referred to as an ‘oncostatic organ’”
Age-associated changes of the pineal gland
The absence of a blood–brain barrier allows the pineal gland to freely accumulate minerals, such as calcium, and trace elements, including zinc, cobalt, fluoride and selenium (Chlubek and Sikora, 2020). The progressive accumulation of minerals leads to the formation of corpora arenacea (‘brain sand’). Eventually, these mineral aggregates enlarge to form pineal concretions up to 1mm in size; this usually leads to a gradual hardening as the pineal gland progressively becomes calcified with age (Sergina et al 2018). Pineal calcification is readily visible on X-ray and is of use clinically as an anatomical landmark during various forms of brain imaging.
Calcification of the pineal gland is rare in infancy, being found in only around 1% of children under the age of 6 years; however, it quickly rises to around 39% between the ages of 8-14 years (Doyle and Anderson, 2006). As calcification progresses, the volume of active pineal tissue decreases and the secretion of melatonin is reduced. This is thought to, at least partially, explain age-associated changes to sleep patterns such as poor-quality sleep and insomnia (Song, 2019). More worryingly, melatonin is recognised as an important neuroprotective agent and extensive pineal calcification and reduced melatonin secretion has been associated with an increased risk of neurodegenerative disorders, particularly Alzheimer’s disease (Tan et al, 2018).
Cysts (usually benign fluid-filled sacs) are present in around 25-40% of pineal glands (Gheban et al, 2019). Most are small and asymptomatic, and usually only discovered during brain imaging. Cysts that reach larger sizes of between 7mm and 4cm may become symptomatic but, fortunately, are rare. Occasionally, large pineal cysts can weaken blood vessels, potentially resulting in sudden death through intracystic haemorrhage (Gheban et al, 2019).
Thymus gland
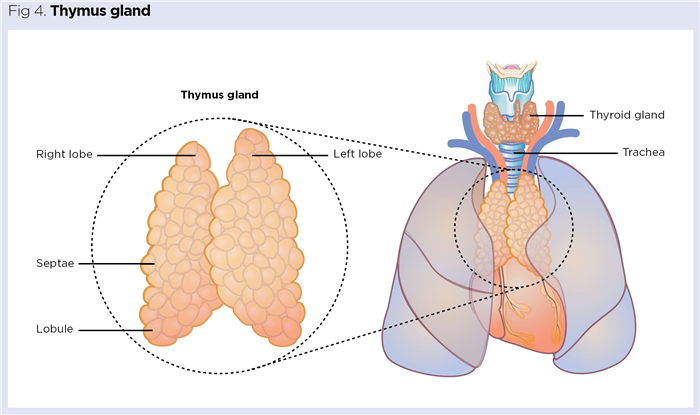
The thymus is a small, delicate bilobed gland located below the manubrium (upper portion of the sternum). It is usually pinkish grey and resides in the mediastinum (space between the lungs) resting on the superior portion of the heart (Fig 4). The gland is protected by a thin outer capsule and each lobe is composed of multiple lobules of around 2-3mm held together by loose connective tissue (Nigam and Knight, 2020).
The thymus is a primary lymphoid organ recognised as having both immune and endocrine functions. Its major immune role is to act as the site where T lymphocytes (T cells) mature before being released into the general circulation. T cells play many diverse immune roles in specific immunity. Most famously, they function as ‘T-helper’ cells, helping B lymphocytes to produce antibodies that mark out pathogens for subsequent destruction. Other populations of T cells include suppressor T cells, which dampen down immune responses, and cytoxic T cells, which target and destroy malignant and virally infected cells.
The thymus gland also helps programme immune cells to recognise ‘self’ antigens (the body’s own antigens) via a process termed ‘thymic education’; this is essential to minimise the body’s immune responses against its own cells and tissues, which could otherwise lead to autoimmune disease (Nigam and Knight, 2020).
Endocrine function
Internally, each lobule of the thymus consists of multiple follicles, composed of a framework of epithelial cells and a population of T cells in varying states of maturation. The thymus gland secretes a range of peptide hormones synthesised by the thymic epithelial cells, including thymulin, thymopoietin, thymic humoral factor and thymosin (Rezzani et al, 2020).
The exact mechanisms by which thymic hormones exert their effects remain poorly understood, but they are known to be essential to normal immune function because they facilitate development and maturation of T cells in the thymic follicles. This ensures competent, functional T cells are released into the circulation where they can participate in effective immune responses. Thymic hormones are used therapeutically to treat many infections, malignancies and certain autoimmune diseases (Severa et al, 2019).
Thymic involution
The thymus gland progressively atrophies (shrinks) with age. Before puberty, it is relatively large, weighing around 40g. By 20 years of age, however, it is typically 50% smaller than at birth and, by the age of 60 years, it is around a sixth of its original size (Bilder, 2016). This progressive atrophy is referred to as thymic involution and is thought to be associated with immunosenescence, as the aged thymus releases fewer T cells into the circulation (Rezzani et al, 2020). Immunosenescence is a major feature of ageing and results in declining immune responses, which can increase the risk of infections and malignancies.
Melatonin and immune responses
Melatonin is known to have a stimulatory effect on both non-specific (innate) immunity and specific immune responses. Reduced melatonin secretion leads to a progressive decrease in the weights of the spleen and thymus gland, and a decrease in the numbers of circulating lymphocytes. Most of these negative immunological effects are reversed by administration of exogenous melatonin (Carrillo-Vico et al, 2005).
Melatonin ‘upregulates’ many aspects of non-specific immunity, including:
- Increasing haematopoiesis (manufacture of blood cells);
- Elevating numbers of cancer-fighting natural killer (NK) cells;
- Enhancing the pathogen-killing ability of leukocytes, such as phagocytic macrophages.
Administrating exogenous melatonin in patients with infections is reported to both reduce the duration of infection and improve patient outcomes (Vinther and Claesson, 2015). In addition to pineal-derived melatonin, many immunologically reactive cells, such as mast cells and lymphocytes, are themselves able to synthesise and release melatonin. This locally generated melatonin is then able to participate in the modulation of immune responses. Although melatonin is generally regarded as an upregulator of immune function, it also suppresses the production of many pro-inflammatory mediators, thereby dampening inflammatory responses when necessary (Csaba, 2013).
Melatonin directly influences the activity of the thymus gland by inducing the secretion of thymic hormones, such as thymosin and thymulin. This is thought to enhance the differentiation of T cells in the thymus, thereby enhancing T cell mediated immune responses (Vinther and Claesson, 2015). Conversely, hormones generated by the thymus are able to circulate in the plasma and modulate the secretion of melatonin by the pineal gland (Csaba, 2013).
This crosstalk between the two glands, and the documented link between the production of melatonin and activity of the thymus gland, has led to the speculative existence of a thymus–pineal axis (Rezzani et al, 2020). Additionally, the widespread and diverse influences of melatonin on immunity is frequently referred to as the immune–pineal axis (Markus et al, 2018).
Melatonin and malignancy
The positive effect of melatonin on NK-cell numbers is thought to contribute to its ability to enhance immune responses against a wide range of malignancies. Melatonin is reported to inhibit the growth and spread of several tumour cell lines, including those associated with melanoma and ovarian, breast, liver, colorectal and prostate cancers (Vinther and Claesson, 2015). Indeed, melatonin appears to be so effective in inhibiting the proliferation of certain tumour cells that the pineal gland has been routinely referred to as an ‘oncostatic organ’ (Kajdaniuk et al, 1999).
Exogenous melatonin is also a useful adjunct to many forms of radiotherapy and chemotherapy; it not only enhances the effectiveness of the treatment, but also helps to minimise some of the unwanted, adverse side-effects (Talib et al, 2021).
Conclusion
This article has explored the anatomy, physiology and function of the pineal and thymus glands. Part 6 will focus on the pancreas, liver and gastrointestinal tract.
Key points
- The pineal gland is a neuroendocrine gland in the brain, which produces the hormone melatonin
- Melatonin is key to synchronising the body’s biological rhythms and has an influence on immunity
- The thymus has both immune and endocrine functions, and produces hormones that are essential to normal immune function
- An association between the activities of the thymus and pineal glands has led to the postulation of a thymus–pineal axis
- The pineal and thymus glands both undergo significant age-associated changes thought to be associated with declining physiological function and disease
Also in this series
Afroz H et al (2014) Different shapes of the Human pineal gland: a study on 60 autopsy cases. Journal of Dhaka Medical College; 23: 2, 211-214.
Bilder GE (2016) Human Biological Aging: From Macromolecules to Organ Systems. Wiley Blackwell.
Carrillo-Vico A et al (2005) A review of the multiple actions of melatonin on the immune system. Endocrine: 27: 2, 189-200.
Challet E (2015) Keeping circadian time with hormones. Diabetes, Obesity and Metabolism; 17: Suppl 1, 76–83.
Chlubek D, Sikora M (2020) Fluoride and pineal gland. Applied Sciences; 10: 8, 2885.
Cipolla-Neto J, Amaral FG (2018) melatonin as a hormone: new physiological and clinical insights. Endocrine Reviews; 39: 6, 990-1028.
Csaba G (2013) The pineal regulation of the immune system: 40 years since the discovery. Acta Microbiologica et Immunologica Hungarica; 60: 2, 77–91.
Douma LG, Gumz ML (2018) Circadian clock-mediated regulation of blood pressure. Free Radical Biology and Medicine; 119: 108-114.
Doyle AJ, Anderson GD (2006) Physiologic calcification of the pineal gland in children on computed tomography: prevalence, observer reliability and association with choroid plexus calcification. Academic Radiology; 13: 7, 822-826.
Emet M et al (2016) A review of melatonin, its receptors and drugs. The Eurasian Journal of Medicine; 48: 2, 135–141.
Gheban BA et al (2019) The morphological and functional characteristics of the pineal gland. Medicine and Pharmacy Reports; 92: 3, 226-234.
Grima M et al (2017) Molecular mechanisms of the sleep wake cycle: therapeutic applications to insomnia. Xjenza Online; 5, 87-97.
Ibañez Rodriguez MP et al (2016) Cellular Basis of Pineal Gland Development: Emerging Role of Microglia as Phenotype Regulator. PLoS ONE; 11: 11, e0167063.
Kajdaniuk D et al (1999) Oncostatic effect of melatonin action – facts and hypotheses Medical Science Monitor; 5: 2, RA350-356.
Markus RP et al (2018) Immune-pineal axis – acute inflammatory responses coordinate melatonin synthesis by pinealocytes and phagocytes. British Journal of Pharmacology; 175: 16, 3239-3250.
Nigam Y, Knight J (2020) The lymphatic system 2: structure and function of the lymphoid organs. Nursing Times [online]; 116: 11, 44-48.
Omar SH, Saba N (2010) Melatonin, receptors, mechanism, and uses. Systematic Reviews in Pharmacy; 1: 2, 158-171.
Patel S et al (2020) Revisiting the pineal gland: a review of calcification, masses, precocious puberty, and melatonin functions. International Journal of Neuroscience; 130: 5, 464-475.
Pateras E (2020) Blue light blocking ophthalmic lenses and their benefits: a review. Journal of Materials Science Research and Reviews; 5: 3, 13-20.
Rafique N et al (2020) Effects of mobile use on subjective sleep quality. Nature and Science of Sleep; 12: 357–364.
Rezzani R et al (2020) Thymus-pineal gland axis: revisiting its role in human life and ageing. International Journal of Molecular Sciences; 21: 22, 8806.
Sergina SN et al (2018) [Taxonomic and ethnical dispersion of phenomenon of pineal concretions in the gerontological context]. Advances in Gerontology; 31: 6, 913-924.
Severa M et al (2019) Thymosins in multiple sclerosis and its experimental models: moving from basic to clinical application. Multiple Sclerosis and Related Disorders; 27: 52-60.
Shechter A et al (2018) Blocking nocturnal blue light for insomnia: a randomized controlled trial. Journal of Psychiatric Research; 96, 196–202.
Song J (2019) Pineal gland dysfunction in Alzheimer’s disease: relationship with the immune-pineal axis, sleep disturbance, and neurogenesis. Molecular Neurodegeneration; 14: 28.
Talib WH et al (2021) Melatonin in cancer treatment: current knowledge and future opportunities. Molecules; 26: 2506.
Tan DX et al (2018) Pineal calcification, melatonin production, aging, associated health consequences and rejuvenation of the pineal gland. Molecules; 23: 2, 301.
Tordjman S et al (2017) Melatonin: pharmacology, functions and therapeutic benefits. Current Neuropharmacology; 15: 3, 434-443.
Vinther AG, Claesson MH (2015) The influence of melatonin on immune system and cancer. International Journal of Cancer and Clinical Research; 2: 024.
Zhao D et al (2019) Melatonin synthesis and function: evolutionary history in animals and plants. Frontiers in Endocrinology; 10: 249.
Zisapel N (2018) New perspectives on the role of melatonin in human sleep, circadian rhythms and their regulation. British Journal of Pharmacology; 175: 16, 3190–3199.