Endocrine system 2: hypothalamus and pituitary gland
The endocrine system comprises glands and tissues that produce hormones for regulating and coordinating vital bodily functions. This article, the second in an eight-part series, looks at the hypothalamus and pituitary gland
Abstract
The endocrine system consists of glands and tissues that produce and secrete hormones to regulate and coordinate vital bodily functions. This article, the second in an eight-part series on the endocrine system, explores the anatomy and physiology of the hypothalamus and pituitary gland, and how they work together to regulate and coordinate vital physiological processes in the body through hormonal action. It shows how many of the actions initiated by the hypothalamus are mediated through hormonal secretions produced by the pituitary gland beneath it.
Citation: Bayram-Weston Z et al (2021) Endocrine system 2: hypothalamus and pituitary gland. Nursing Times [online]; 117: 6, 49-53.
Authors: Zubeyde Bayram-Weston is senior lecturer in biomedical science; Maria Andrade is honorary lecturer in biomedical science; John Knight is associate professor in biomedical science; all at College of Human and Health Sciences, Swansea University.
- This article has been double-blind peer reviewed
- Scroll down to read the article or download a print-friendly PDF here (if the PDF fails to fully download please try again using a different browser)
- Click here to see other articles in this series
The first article in this eight-part series on the endocrine system gave an overview of the nature of endocrine glands and highlighted the role of hormones as chemical signals that help maintain the homeostatic balance essential to health; the remaining articles will each explore different major endocrine glands and tissues. This article examines the anatomy and physiology of the hypothalamus and pituitary gland, which lie in the cranial cavity of the skull.
The hypothalamus
The hypothalamus is located at the base of the brain just below the thalamus. A small but vital region of the brain, it is roughly the size of an almond and weighs around 4g (Saper and Lowell, 2014). It accounts for
The hypothalamus is part of the limbic system, a region of the brain that also includes the thalamus, amygdala, hippocampus and cingulate gyrus. The limbic system is well developed in all higher vertebrates and plays a key role in emotional responses, long-term memory, sense of smell (olfaction) and the acquisition of new skills, as well as contributing to a range of behavioural responses (Wróbel, 2018). The hypothalamus is the location of the thermoregulatory centre, which regulates body temperature (VanPutte et al, 2017); it plays an essential role in water balance, regulation of blood pressure and the sensations of thirst and hunger.
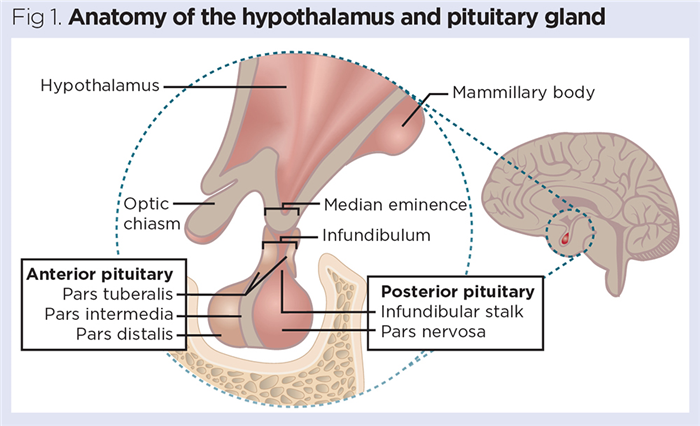
This article focuses on the endocrine functions of the hypothalamus and its role as the key link between the nervous and endocrine systems. The hypothalamus is connected directly to the pituitary gland via a thin stalk, called the infundibulum (Fig 1). Many actions initiated by the hypothalamus are mediated through secretions produced by the pituitary gland beneath it.
The pituitary gland
The pituitary gland is a pea-sized gland that is typically around 0.8-1.0cm in diameter and weighs around 500mg. It resides in the sella turcica (Turkish saddle), a protective pocket in the sphenoid bone of the skull (Fig 1). Increased pituitary size is often indicative of endocrine pathologies, particularly tumours of the pituitary (De Sousa et al, 2015). The pituitary gland comprises two regions (Fig 1):
- Posterior pituitary (neurohypophysis) – neural tissue extends from the hypothalamus through the infundibulum into a larger, bulbous region called the pars nervosa; this forms the bulk of the posterior pituitary;
- Anterior pituitary (adenohypophysis) – derived from the epithelial tissue of the embryonic oral cavity.
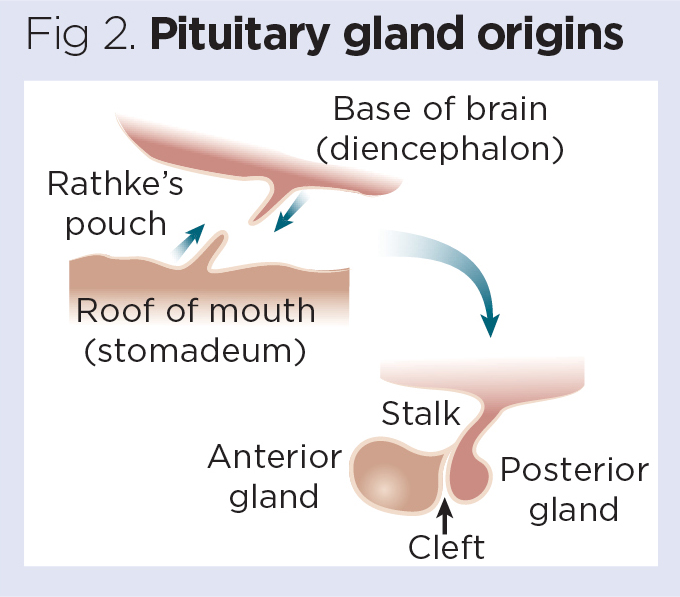
During embryonic development, the roof of the mouth bulges upwards (invaginates) to form a tiny, bubble-like structure known as Rathke’s pouch, which then fuses with the posterior portion of the pituitary gland (Fig 2). Failure of this process to occur normally may lead to an abnormal pituitary structure or the formation of cysts and clefts (Babu et al, 2013).
The anterior pituitary accounts for approximately 70-80% of the total mass of the gland and includes two major parts:
A third (intermediate) region of the pituitary gland is often recognisable; this is known as the pars intermedia and is usually present as a thin band of tissue that marks the point where the anterior and posterior pituitaries fuse (Ilahi and Ilahi, 2020).
Hormones of the posterior pituitary
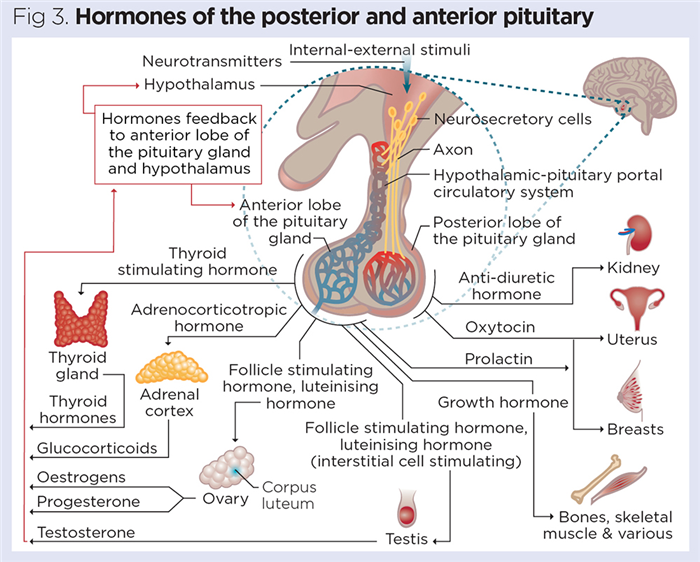
Two major hormones are released from the posterior pituitary:
These hormones are synthesised in the cell bodies of neurons in the hypothalamus and transported down the axons of the neurons running through the infundibulum. ADH and oxytocin are concentrated and stored in the pars nervosa (Fig 3), before being released into the blood when required. Both are peptide hormones and, as they are produced by neurons, they are often called neuropeptides.
Antidiuretic hormone
ADH plays a vital role in regulating fluid balance and blood pressure. Specialised osmoreceptors located in the hypothalamus continually monitor the solute concentration of the blood. When the body loses water (for example, through sweating during exercise or following vomiting and diarrhoea) dehydration may occur and the plasma solute concentration rises. This is detected by the hypothalamic osmoreceptors, which initiate the release of ADH from the posterior pituitary.
ADH primarily acts on the kidneys, increasing the volume of fluid absorbed from the renal filtrate back into the blood. This reduces the volume of urine produced (hence the name antidiuretic hormone), resulting in the urine being darker and more highly concentrated. By increasing fluid reabsorption back into the blood, ADH helps normalise the solute concentration of the blood (VanPutte et al, 2017).
ADH is also released after a drop in blood volume or pressure. By promoting water reabsorption in the kidney, ADH increases blood volume, which then starts to increase blood pressure. This normalisation of blood pressure is further enhanced by ADH acting as a powerful vasopressor (which promotes the constriction of blood vessels). ADH-induced vasoconstriction, particularly in the peripheral arterioles (small arteries), further increases and normalises blood pressure (Kanbay et al, 2019). As a result, ADH is also known as vasopressin, particularly in the United States.
Reduced secretion of ADH can lead to diabetes insipidus (DI). Patients with DI cannot concentrate their urine, resulting in polyuria. Large volumes of urine (3-20L/day) are usually produced; if not treated, this can lead to severe dehydration.
DI is rare, affecting around 1 in 25,000 people; two major types are recognised:
- Neurogenic or central DI is caused by the undersecretion (hyposecretion) of ADH by the posterior pituitary. This is most often due to trauma (commonly head injuries), tumours affecting the hypothalamus or pituitary or, more rarely, infections;
- Nephrogenic DI is a rarer form, in which patients usually have normal ADH synthesis and secretion, but their kidneys are insensitive to the effects of ADH – most commonly due to kidney disease or drug-induced kidney damage (Kalra et al, 2016).
DI requires careful management. Initially patients may be severely dehydrated, feel nauseous and shivery, and experience headache; careful monitoring of water intake and urine output, with ongoing assessment of urine and blood concentration, is essential. Neurogenic DI is usually treated with desmopressin, a synthetic analogue of ADH that acts on the kidneys in the same way to concentrate the urine and increase blood volume. Treatment of nephrogenic DI is more complex and depends on the underlying cause of the disease (The Pituitary Foundation, 2016).
Oxytocin
Oxytocin is released into the blood at high concentration towards the end of the gestational period and initiates parturition (childbirth) by stimulating contractions of the myometrium (muscular layer of the uterus). Oxytocin secretion is regulated by a positive feedback mechanism, whereby increased oxytocin stimulates more-powerful myometrial contractions, which in turn stimulate the release of more oxytocin (VanPutte et al, 2017). This is possible because the uterine wall has receptors that monitor the strength of myometrial contractions and generate nerve impulses (action potentials) that are relayed back to the hypothalamus.
Oxytocin also stimulates the ‘letdown reflex’ in lactating mothers; here the smooth muscle linings of the milk ducts in the breast contract, making milk available to the baby during suckling. Again, this is regulated by positive feedback, with the mechanical stimulation of the baby’s suckling action triggering the release of more oxytocin (Osilla and Sharma, 2020).
Oxytocin is often referred to as ‘the love hormone’ because it plays an important role in promoting mother/baby bonding; it is also thought to facilitate pair bonding between partners. Evidence is also emerging that oxytocin has other psychological effects, such as reducing anxiety, and promoting maternal behaviour (Parmar and Malik, 2017).
Hormones of the anterior pituitary
The anterior pituitary produces a far greater range of hormones than the posterior pituitary. The anterior pituitary’s role in producing a variety of stimulating hormones that regulate the activity of many other endocrine glands is why the pituitary is often referred to as the ‘master gland’. As explained below, this is a misnomer: the release of these stimulating hormones is governed by hormones released from the hypothalamus, which ultimately acts as the true primary orchestrator of endocrine function.
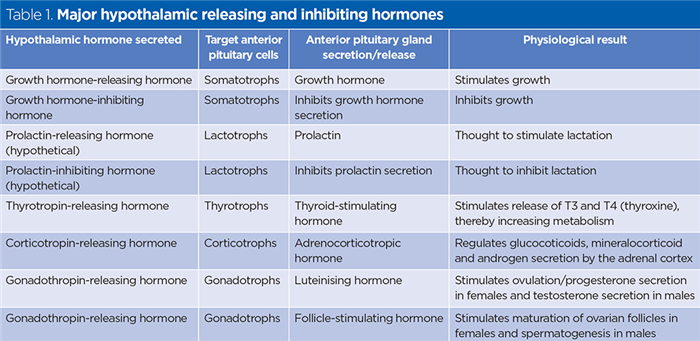
The anterior pituitary’s cells are usually classified into five major types based on the nature of their secretions. These are listed below with their hormonal secretions:
- Somatotrophs – somatotropin or growth hormone (GH);
- Lactotrophs – prolactin;
- Thyrotrophs – thyroid-stimulating hormone (TSH);
- Corticotropths – adrenocorticotropic hormone (ACTH) and melanocyte-stimulating hormone (MSH);
- Gonadotrophs – follicle-stimulating hormone (FSH) and luteinising hormone (LH).
Growth hormone
As its name suggests, the primary function of GH is to promote bodily growth. Most famously, GH promotes the widening of the growth plates in the epiphyses of the long bones of the skeleton, which results in elongation of the major bones of the arms and legs, progressively increasing height. GH also enhances amino acid uptake from the blood into cells, increasing the rate of protein synthesis in tissues such as muscle; this is why it is known as an anabolic hormone.
Thyroid hormones T3 and T4 (thyroxine), which regulate metabolism, are necessary for GH to exert its effects efficiently. The anabolic effects of GH are also enhanced by the presence of other anabolic hormones such as testosterone. As well as promoting bone and muscle growth, GH also stimulates the growth of many of the major internal organs (Devesa et al, 2016).
GH secretion is regulated by two hormones produced by the hypothalamus:
- Growth hormone-releasing hormone stimulates the release of GH;
- Growth hormone-inhibiting hormone (GHIH) acts antagonistically to inhibit the release of GH (Table 1).
Deficiency of GH during childhood may result in pituitary dwarfism; this is characterised by below-average growth and, commonly, an underdeveloped bridge of the nose and prominent forehead. Unlike achondroplastic dwarfism (a genetic disorder), pituitary dwarfism, although associated with reduced height, is characterised by normal bodily proportions. Recombinant human GH is available to treat children who are deficient in GH. It is usually injected subcutaneously once a day, and growth rate and potential side-effects then carefully monitored (Rose et al, 2014).
Elevated secretion of GH in childhood often leads to gigantism, in which rapid growth of the long bones can result in an adult height of >2.4m. Elevated secretion of GH in adults, after their epiphyseal growth plates have fused, can lead to acromegaly, in which the hands, feet and some facial features (particularly the lower jawbone) can grow abnormally large and usually out of normal proportion (de Herder, 2009).
Prolactin
Prolactin (lactogenic hormone) initiates milk secretion (lactation) in breast tissue. By itself, prolactin has only a weak effect, but during pregnancy prolactin levels increase and it acts synergistically with other hormones – including oestrogens, progesterone and cortisol – to promote the enlargement and engorgement of the breasts in preparation for lactation (Suarez et al, 2015).
It has been hypothesised that the release of prolactin is regulated and fine-tuned by the antagonistic actions of a prolactin-releasing hormone and a prolactin-inhibiting hormone, both of which are thought to be produced by the hypothalamus (Table 1).
Tropic hormones
Tropic hormones have a stimulating effect on other endocrine glands, inducing the synthesis and secretion of the target hormone(s). Four major tropic hormones are synthesised and secreted by the anterior pituitary, as described below.
Thyroid-stimulating hormone (thyrotrophin)
TSH stimulates the thyroid gland to secrete the iodine-containing hormones T3 and T4. These are primarily responsible for regulating metabolism, with T3 being the more potent. Most cell types in the body have internal receptors for T3 and T4. These hormones are also vital for growth and development, and play key roles in the normal functioning of the cardiovascular, respiratory, skeletal and central nervous systems.
The release of TSH is regulated by thyrotropin-releasing hormone, which is produced by the hypothalamus (Table 1). The fine tuning of T3 and T4 release is regulated by negative feedback, through the sequential secretions of the hypothalamus, anterior pituitary and thyroid gland (Fitzgerald and Bean, 2018). This hormonal cascade is referred to as the hypothalamic-pituitary-thyroid (HPT) axis and will be explored in detail in part 3 of this series.
Adrenocorticotrophic hormone (adrenocorticotropin)
ACTH primarily regulates the production and secretion of cortisol from the adrenal cortex (outer portion of the adrenal gland). Cortisol is a long-term stress hormone and a steroidal hormone synthesised from cholesterol. It is referred to as a glucocorticoid because it is produced by the adrenal cortex and influences the concentration of glucose in the blood (VanPutte et al, 2017). Following periods of chronic stress (including classic biological stressors such as starvation or physical injury), the hypothalamus releases corticotropin-releasing hormone. This initiates the release of ACTH from the anterior pituitary and, subsequently, stimulates the release of cortisol from the adrenal cortex (Table 1).
Cortisol plays a key role in regulating metabolism and, during periods of food deprivation, stimulates the breakdown of protein and fat to generate glucose for use as fuel in glucose-dependent tissues, such as the brain. This process is called gluconeogenesis (literally, the creation of new glucose). Cortisol also influences the sleep/wake cycle, mood and behaviour, and has potent anti-inflammatory/immunosupressant properties (Kandhalu, 2013).
ACTH also helps to regulate the release of other steroid hormones produced by the adrenal cortex, including aldosterone (which regulates the concentration of sodium and potassium in the blood) and the group of testosterone-like hormones known as androgens (Gallo-Payet, 2016). The complex interplay between the hypothalamus, anterior pituitary and the adrenal cortex is referred to as the HPT axis and will be examined in detail in part 4 of this series.
ACTH is also part of the melanocortin group of hormones, which influence skin pigmentation (see below).
MSH is synthesised by the pars intermedia region of the pituitary gland. Although this region marks the boundary where the anterior and posterior portions of the pituitary gland fuse, it is generally considered part of the anterior pituitary. The pars intermedia atrophies (shrinks) with age and, in adults, may only be present as a vestigial remnant or, in some cases, is not recognisable at all. MSH exists in a range of structurally similar forms known as melanocortins, which are all small peptides.
As implied by its name, MSH stimulates the pigment-producing cells (melanocytes) in the epidermis to release the dark pigment known as melanin, which is largely responsible for skin colour. All races are thought to have similar numbers of melanocytes in their epidermis; it is the relative activity of these cells and the amount of melanin they synthesise and release that ultimately determines skin colour.
Melanocytes can synthesise MSH when exposed to the ultraviolet (UV) light in sunlight (Tsatmali et al, 2002). This is essential to protect the actively dividing cells of the epidermis from the harmful effects of UV, known to cause DNA damage that can lead to mutations and, potentially, skin cancers. Melanin is excellent at absorbing UV wavelengths of light and, as it accumulates in the epidermis the skin, darkens and develops a protective suntan.
During pregnancy, levels of MSH tend to increase, which, together with changes to the sex hormones oestrogen and progesterone, often leads to hyper-pigmentation around the eye sockets, cheekbones, lips and forehead. This is known as melasma or ‘the mask of pregnancy’; these pigmented areas usually fade gradually after childbirth (Costin and Birlea, 2006).
ACTH (described above) is another hormone that can influence skin pigmentation through the direct stimulation of melanocytes. This is particularly true of certain forms of Cushing’s syndrome, in which excess ACTH often causes regions of dark, hyperpigmented skin; this will be discussed further in part 4 of this series.
These act on the gonads (testes and ovaries) to stimulate the production of sex hormones and sperm or ova in males and females respectively (see below). The main gonadrotrophins are FSH and LH; the release of both is regulated by gonadotropin-releasing hormone, which is produced by the hypothalamus (Table 1).
In females, each month FSH initiates the development of immature follicles in the ovaries. As each follicle enlarges, it secretes the female sex hormone oestrogen, before maturing into a Graafian follicle, a fluid-filled, pressurised sac containing a mature ovum (egg), primed and ready to rupture. Ovulation is triggered by LH, which initiates rupturing of the follicle and ovarian wall; this explosive event propels the ovum into its adjacent fallopian tube.
Following ovulation, the remnants of the Graafian follicle collapse to form a structure known as the corpus luteum (yellow body). This produces the second major female sex hormone, progesterone, which maintains the integrity of the endometrial lining of the uterus to allow for the implantation of a fertilised ovum (VanPutte et al, 2017).
Despite their names being reflective of the role played in the female ovarian cycle, FSH and LH also play crucial roles in male reproductive physiology. FSH is essential in stimulating spermatogenesis, where diploid cells (containing 46 chromosomes) undergo meiotic division to produce vast numbers of haploid spermatozoa (each containing 23 chromosomes).
FSH also stimulates the activity of Sertoli cells (‘nurse’ cells) in the testes; these provide nutrition to the developing spermatozoa, allowing maturation into viable gametes that are capable of fertilisation. LH stimulates the interstitial cells (Leydig cells) of the testes to synthesise and release the male sex hormone testosterone (Babu et al, 2004). This powerful anabolic steroid stimulates skeletal muscle development, growth of facial and body hair, expansion of the larynx (causing the deepening of the voice) and spermatogenesis, and is largely responsible for the male sex drive.
The role of the gonadotropins and male and female sex hormones will be discussed further in part 7 of this series.
Role of the hypothalamus
The pituitary gland is often referred to as the master gland but, in fact, it plays more of a ‘middle-management’ role; many of its actions are directed by the hypothalamus.
Hypothalamic nuclei and hypothalamic-pituitary portal system
The hypothalamus contains discrete, organised clusters of neurons called the hypothalamic nuclei, which synthesise the hypothalamic releasing and inhibiting hormones that regulate the activity of the anterior pituitary. Both the hypothalamus and pituitary gland are highly vascularised and have a dedicated network of blood vessels called the hypothalamic-pituitary portal system, which ensures rapid and efficient delivery of the releasing and inhibiting hormones from the hypothalamus to the anterior pituitary below (Bear et al, 2021).
Secretion of the hypothalamic releasing and inhibiting hormones is determined by multiple sensory inputs, which continually monitor the changing physiological status of the body. Multiple parameters monitored continuously and in real time include temperature, pH, solute concentrations and current levels of circulating hormones. The hypothalamus functions as the key bridge between the nervous and endocrine systems, but many of the interactions between the two remain poorly understood.
Table 1 summarises the key hormones of the hypothalamus and pituitary, and their relationships. Some of the better-studied interactions between the hypothalamus, pituitary and peripheral endocrine glands (such as the HPT axis and hypothalamic-pituitary-adrenal axis) will be explored later in this series. Part 3 focuses on the thyroid and parathyroid glands.
Key points
- The hypothalamus and pituitary gland both lie in the cranial cavity of the skull
- Two major hormones released by the posterior pituitary gland are antidiuretic hormone and oxytocin
- The anterior pituitary gland produces several stimulating hormones that regulate the activity of other endocrine glands
- Although the pituitary gland is often referred to as the master gland, many of its actions are directed by the hypothalamus
- Clusters of neurons in the hypothalamus synthesise releasing and inhibiting hormones that regulate the activity of the anterior pituitary
Also in this series
References
Babu R et al (2013) Symptomatic Rathke’s cleft cyst with a co-existing pituitary tumor; brief review of the literature. Asian Journal of Neurosurgery; 8: 4, 183-187.
Babu SR et al (2004) Evaluation of FSH, LH and testosterone levels in different subgroups of infertile males. Indian Journal of Clinical Biochemistry; 19: 1, 45-49.
Bear MH et al (2021) Neuroanatomy, Hypothalamus. StatPearls Publishing.
Costin G-E, Birlea S-A (2006) What is the mechanism for melasma that so commonly accompanies human pregnancy? International Union of Biochemistry and Molecular Biology Life; 58: 1, 55-57.
De Herder WW (2009) Acromegaly and gigantism in the medical literature. Case descriptions in the era before and the early years after the initial publication of Pierre Marie (1886). Pituitary; 12: 3, 236-244.
De Sousa SMC et al (2015) Pituitary hyperplasia: case series and literature review of an under-recognised and heterogeneous condition. Endocrinology, Diabetes and Metabolism Case Reports; 2015: 150017.
Devesa J et al (2016) Multiple effects of growth hormone in the body: is it really the hormone for growth? Clinical Medicine Insights: Endocrinology and Diabetes; 9: 47-71.
Fitzgerald SP, Bean NG (2018) Thyroid stimulating hormone (TSH) autoregulation reduces variation in the TSH response to thyroid hormones. Temperature; 5: 4, 380-389.
Gallo-Payet N (2016) Adrenal and extra-adrenal functions of ACTH. Journal of Molecular Endocrinology; 56: 4, T135-T156.
Kalra S et al (2016) Diabetes insipidus: the other diabetes. Indian Journal of Endocrinology and Metabolism; 20: 1, 9-21.
Kanbay M et al (2019) Antidiuretic hormone and serum osmolarity physiology and related outcomes: what is old, what is new, and what is unknown? Journal of Clinical Endocrinology and Metabolism; 104: 11, 5406-5420.
Kandhalu P (2013) Effects of cortisol on physical and psychological aspects of the body and effective ways by which one can reduce stress. Berkeley Scientific Journal; 18: 1, 14-16.
Osilla EV, Sharma S (2020) Oxytocin. StatPearls Publishing.
Parmar P, Malik S (2017) Oxytocin: the hormone of love. International Organization of Scientific Research Journal of Pharmacy and Biological Sciences; 12: 6, 1-9.
Pereira Suarez AL et al (2015) Prolactin in inflammatory response. Advances in Experimental Medicine and Biology; 846: 243-264.
The Pituitary Foundation (2016) Diabetes Insipidus. The Pituitary Foundation.
Rose SR et al (2014) Growth hormone therapy guidelines: clinical and managed care perspectives. American Journal of Pharmacy Benefits; 6: 5, e134-e146.
Saper CB, Lowell BB (2014) The hypothalamus. Current Biology; 24: 23, R1111-1116.
Tsatmali M et al (2002) Melanocyte function and its control by melanocortin peptides. Journal of Histochemistry and Cytochemistry; 50: 2, 125-133.
VanPutte CL et al (2017) Seeley’s Anatomy and Physiology. McGraw-Hill.
Wróbel G (2018) The structure of the brain and human behaviour. Pedagogy and Psychology of Sport; 4: 1, 37-51.